
How are the following conversions carried out?
I) tert-butyl bromide to isobutyl bromide
Answer
570.3k+ views
Hint: For the, of tert-butyl bromide do the dehydrohalogenation reaction. Dehydrohalogenation reaction is the reaction in which a hydrogen and halide ion is eliminated as hydrogen halide.
Complete answer:
So here we have to do an organic conversion reaction in which we have to convert tert-butyl bromide to isobutyl bromide, both contains four atoms, only there is difference in the position of halide atom i.e. the Br atom to which C atom it is attached to. Both the compounds are isomers of 1- bromobutane.
First let's draw the structures of the molecule that should undergo conversion i.e., tert-butyl bromide
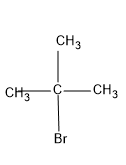
Now we can draw the structure of the product, we should get.
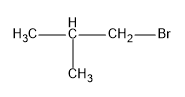
So we know that there is a change in the position of the bromine atom, in the first structure it is attached to the second carbon and in the final product it is attached to the first carbon,
Now let’s see the conversion of the compounds.
First we take alcoholic KOH i.e. KOH in ethanol is made to react with tert-butyl bromide, we know that alcoholic.KOH acts as a strong base and it abstracts beta hydrogen and halide from the saturated reactant and forms an alkene through the elimination reaction.
This reaction is also called the dehydrohalogenation reaction.
Then the obtained intermediate product is allowed to react with HBr in the presence of peroxide. Due to the peroxide effect the nucleophile will attack the least substituted carbon or carbon with more hydrogen and yields the final product i.e. iso-butyl bromide. The conversion reaction is as follows,
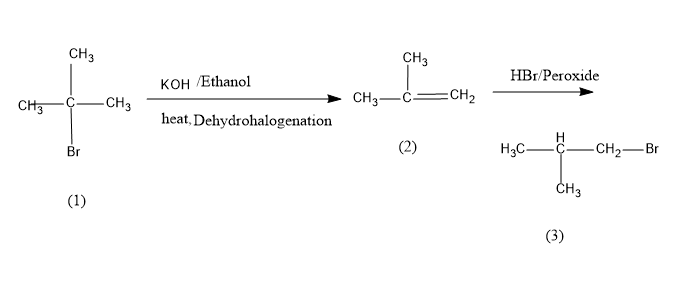
Tert-Butyl bromide is converted into isobutene on dehydrohalogenation which on bromination in presence of peroxide yields iso-butyl bromide or 1-bromo-2-methylpropane,
The IUPAC name of isobutene is 2-methyl propane.
Note: If the bromination is done using HBr in the absence of peroxide, then the Br atom, which is the nucleophile must have attacked the carbon with lower hydrogen atom i.e. most substituted carbon, which is the Markonikov’s rule. Peroxide effect follows the anti-Markovnikov's rule.
Complete answer:
So here we have to do an organic conversion reaction in which we have to convert tert-butyl bromide to isobutyl bromide, both contains four atoms, only there is difference in the position of halide atom i.e. the Br atom to which C atom it is attached to. Both the compounds are isomers of 1- bromobutane.
First let's draw the structures of the molecule that should undergo conversion i.e., tert-butyl bromide

Now we can draw the structure of the product, we should get.

So we know that there is a change in the position of the bromine atom, in the first structure it is attached to the second carbon and in the final product it is attached to the first carbon,
Now let’s see the conversion of the compounds.
First we take alcoholic KOH i.e. KOH in ethanol is made to react with tert-butyl bromide, we know that alcoholic.KOH acts as a strong base and it abstracts beta hydrogen and halide from the saturated reactant and forms an alkene through the elimination reaction.
This reaction is also called the dehydrohalogenation reaction.
Then the obtained intermediate product is allowed to react with HBr in the presence of peroxide. Due to the peroxide effect the nucleophile will attack the least substituted carbon or carbon with more hydrogen and yields the final product i.e. iso-butyl bromide. The conversion reaction is as follows,

Tert-Butyl bromide is converted into isobutene on dehydrohalogenation which on bromination in presence of peroxide yields iso-butyl bromide or 1-bromo-2-methylpropane,
The IUPAC name of isobutene is 2-methyl propane.
Note: If the bromination is done using HBr in the absence of peroxide, then the Br atom, which is the nucleophile must have attacked the carbon with lower hydrogen atom i.e. most substituted carbon, which is the Markonikov’s rule. Peroxide effect follows the anti-Markovnikov's rule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




