
How are the following obtained?
A) Benzoic acid from ethyl benzene
B) Benzaldehyde from toluene.
Answer
556.8k+ views
Hint: We know that there are some oxidizing agents used for oxidation of alkyl groups directly into acid form. There are also certain reagents present which stop the oxidation process at the aldehyde step while some do the full oxidation. Also there are some named reactions which are used for developing a particular group.
Complete step-by-step answer:
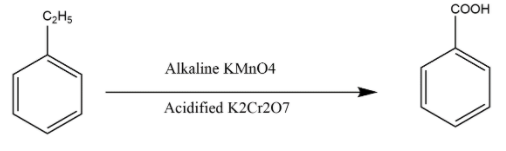
(A)Let’s take the first option where the conversion is in between the ethyl benzene and benzoic acid. So, for converting ethyl benzene into benzoic acid we first have to see the group which changes. In the ethyl benzene there is an ethyl group over benzene while in benzoic acid there is carboxylic acid there, so we can say that the ethyl group gets oxidized to the carboxylic acid group. For this oxidation which is complete and get stop at the last step that is acid and not aldehyde group we can use the strong oxidizing agent like alkaline potassium permanganate \[alkaline\,KMn{O_4}\] or \[acidified\,{K_2}C{r_2}{O_7}\] . Both reagent will do the complete oxidation of the alkyl group into carboxylic group so hence the reaction can be seen as this:
(B) In the second option which is the conversion of toluene to Benzaldehyde, here we can do the Étard’ reaction we have toluene which will react with chromyl chloride and converted into benzaldehyde. The methyl group on benzene changes into the aldehyde group, now in this case the oxidation should stop at the aldehyde stage so, in place of the above reagents we will use this particular named reaction for the conversion.

Note: Reagents used for oxidation and reduction are can be classified into two parts for our understanding, see if you have alcohol so for oxidation of it, it will firstly convert into the aldehyde and then on further oxidation it will changes to carboxylic acid group. Now the oxidation can be stop at any stage as per our requirements for conversion by the reagents used.
Complete step-by-step answer:
(A)Let’s take the first option where the conversion is in between the ethyl benzene and benzoic acid. So, for converting ethyl benzene into benzoic acid we first have to see the group which changes. In the ethyl benzene there is an ethyl group over benzene while in benzoic acid there is carboxylic acid there, so we can say that the ethyl group gets oxidized to the carboxylic acid group. For this oxidation which is complete and get stop at the last step that is acid and not aldehyde group we can use the strong oxidizing agent like alkaline potassium permanganate \[alkaline\,KMn{O_4}\] or \[acidified\,{K_2}C{r_2}{O_7}\] . Both reagent will do the complete oxidation of the alkyl group into carboxylic group so hence the reaction can be seen as this:

(B) In the second option which is the conversion of toluene to Benzaldehyde, here we can do the Étard’ reaction we have toluene which will react with chromyl chloride and converted into benzaldehyde. The methyl group on benzene changes into the aldehyde group, now in this case the oxidation should stop at the aldehyde stage so, in place of the above reagents we will use this particular named reaction for the conversion.

Note: Reagents used for oxidation and reduction are can be classified into two parts for our understanding, see if you have alcohol so for oxidation of it, it will firstly convert into the aldehyde and then on further oxidation it will changes to carboxylic acid group. Now the oxidation can be stop at any stage as per our requirements for conversion by the reagents used.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




