
What are the four structural isomers of the alcohol with molecular formula ${{C}_{4}}{{H}_{9}}OH$?
Answer
497.7k+ views
Hint: We know that the Structural isomers are compounds having the same molecular formula and different structural formulae. This phenomenon is known as structural isomerism.
Complete answer:
As we know that the molecular formula of alcohol with four carbon atoms is ${{C}_{4}}{{H}_{9}}OH.$ Alcohol with four carbon atoms having more than one structural formula which are known as isomers. Alcohols have functional groups \[-OH.\] Alcohols can be classified into primary alcohol, secondary alcohol, and tertiary alcohol.
Firstly, we must start by writing the structure of butanol. Then draw its structure in different forms by changing the substituents and placing alcohol groups in different positions. The structural isomers are the compounds having the same molecular formula but different structural formulae.
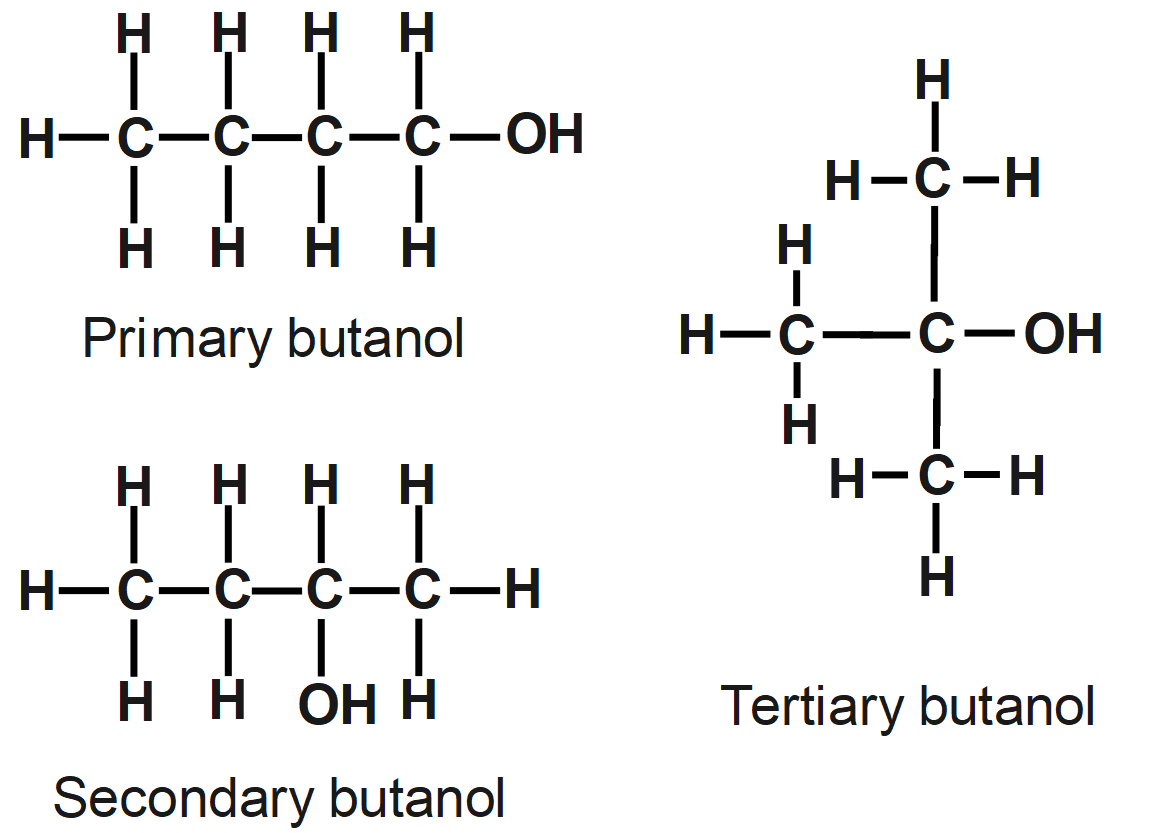
The question says the number of structural isomers of alcohol. Depending on the structural arrangement of atoms, more isomers are ethyl propyl ether, butyl methyl ether, ethyl isopropyl ether, tert-butyl methyl ether, iso-butyl methyl ether, etc. So, the functional group needs to be alcohol only in all the isomers. Butanol can have maximum three structural isomers and the structural isomer of butanol is given as:
Note:
Remember that the structural formula of ${{C}_{4}}{{H}_{9}}OH$ is drawn by changing connectivity of atoms. Different Connectivity of carbon atoms and functional groups will give different constitutional isomers. \[Butan-2-ol\] molecules have a chiral carbon atom, and it is optically active, so it shows optical isomerism.
Complete answer:
As we know that the molecular formula of alcohol with four carbon atoms is ${{C}_{4}}{{H}_{9}}OH.$ Alcohol with four carbon atoms having more than one structural formula which are known as isomers. Alcohols have functional groups \[-OH.\] Alcohols can be classified into primary alcohol, secondary alcohol, and tertiary alcohol.
Firstly, we must start by writing the structure of butanol. Then draw its structure in different forms by changing the substituents and placing alcohol groups in different positions. The structural isomers are the compounds having the same molecular formula but different structural formulae.
The question says the number of structural isomers of alcohol. Depending on the structural arrangement of atoms, more isomers are ethyl propyl ether, butyl methyl ether, ethyl isopropyl ether, tert-butyl methyl ether, iso-butyl methyl ether, etc. So, the functional group needs to be alcohol only in all the isomers. Butanol can have maximum three structural isomers and the structural isomer of butanol is given as:

Note:
Remember that the structural formula of ${{C}_{4}}{{H}_{9}}OH$ is drawn by changing connectivity of atoms. Different Connectivity of carbon atoms and functional groups will give different constitutional isomers. \[Butan-2-ol\] molecules have a chiral carbon atom, and it is optically active, so it shows optical isomerism.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




