
What are the isomers in relation to glucose, galactose and fructose?
Answer
502.2k+ views
Hint: Carbohydrates are sugars containing the atoms of oxygen, hydrogen and oxygen. The general molecular formula of carbohydrates is $ {C_x}{\left( {{H_2}O} \right)_y} $ . The hydrogen atoms will be double the oxygen atoms. Glucose, galactose and fructose are carbohydrates and differ in structure, called structural isomers.
Complete answer:
Glucose is a carbohydrate with the molecular formula of $ {C_6}{H_{12}}{O_6} $ . Galactose is also having a same molecular formula of $ {C_6}{H_{12}}{O_6} $ , fructose has also same molecular formula $ {C_6}{H_{12}}{O_6} $ . But the cyclic structure of glucose, galactose and fructose differ in structures. Hence, these three are called structural isomers.
The glucose is having a hydroxyl group and aldehyde group. The fructose is having a hydroxyl group and ketone group. The galactose has the same configuration as glucose, but there is a difference of hydroxyl group attached direction at $ {C_4} $ carbon.
The cyclic structure of glucose, galactose and fructose are:
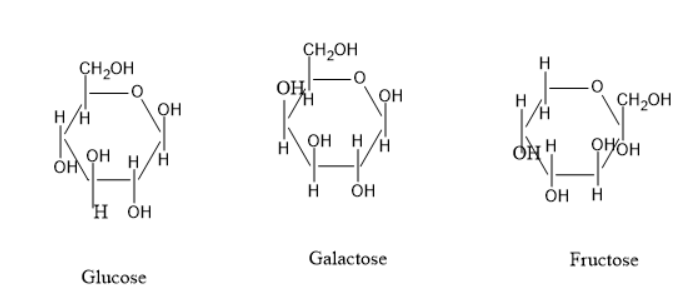
The above three structures have the same molecular formula but there is a difference at the structures, hence these are called structural isomers.
Glucose and galactose have the difference of hydroxyl group position at $ {C_4} $ carbon. The fructose and glucose also have a difference in structure. Thus, these all are structural isomers.
Note:
The glucose and fructose have differences in functional groups in normal structure. But, in cyclic structures there is no difference in functional groups but differ only in structure and can be called structural isomers. The galactose glucose is also having different hydroxyl group positions.
Complete answer:
Glucose is a carbohydrate with the molecular formula of $ {C_6}{H_{12}}{O_6} $ . Galactose is also having a same molecular formula of $ {C_6}{H_{12}}{O_6} $ , fructose has also same molecular formula $ {C_6}{H_{12}}{O_6} $ . But the cyclic structure of glucose, galactose and fructose differ in structures. Hence, these three are called structural isomers.
The glucose is having a hydroxyl group and aldehyde group. The fructose is having a hydroxyl group and ketone group. The galactose has the same configuration as glucose, but there is a difference of hydroxyl group attached direction at $ {C_4} $ carbon.
The cyclic structure of glucose, galactose and fructose are:

The above three structures have the same molecular formula but there is a difference at the structures, hence these are called structural isomers.
Glucose and galactose have the difference of hydroxyl group position at $ {C_4} $ carbon. The fructose and glucose also have a difference in structure. Thus, these all are structural isomers.
Note:
The glucose and fructose have differences in functional groups in normal structure. But, in cyclic structures there is no difference in functional groups but differ only in structure and can be called structural isomers. The galactose glucose is also having different hydroxyl group positions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




