
What are the monomeric repeating units of Nylon 6 and Nylon 6,6?
Answer
585.3k+ views
Hint: The monomers of nylon are a carboxylic acid and amine. The bonds that join these monomeric units are same as that of peptide bonds chemically. In Nylon 6 and Nylon 6,6 these suffix numbers represent the number of carbon atoms present in its monomeric form. Knowledge of the monomeric chemical structure is therefore essential.
Complete step-by-step answer:
We know that a monomer is a molecule that can be bonded to other identical molecules so as to form a polymer for polymeric chain. Nylon 6 and Nylon 6,6 are the two polymers made up of single unit monomers so that by repeating those units we can get these polymers.
Nylon 6 is also known as polycaprolactam. It is a polymer which is formed by ring-opening polymerization of caprolactam. So, we can say that the polymer of nylon 6 is g-caprolactam. During polymerization, an amide bond breaks within each caprolactam molecule. Therefore, the monomeric repeating unit of nylon 6 polymer is \[NH{(C{H_2})_5}CO\] which we derive from caprolactam
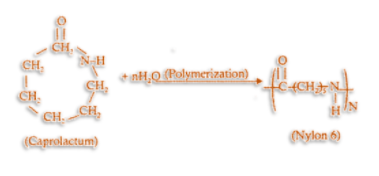 .
.
To prepare nylon 6,6, hexamethylenediamine and adipic acid are added under high pressure so that condensation polymerisation takes place. This clearly tells us that Nylon 6,6 contains amide bonds which are linking the monomeric units and therefore we can call it a polyamide. It is a type of step-growth polymerization, which means it takes place between monomers containing functional groups.
So, the monomers of Nylon 6,6 are hexamethylenediamine and adipic acid which contain functional groups such as amine and carboxylic acid which react in order to produce polymers containing different functionalities than their parent monomers.
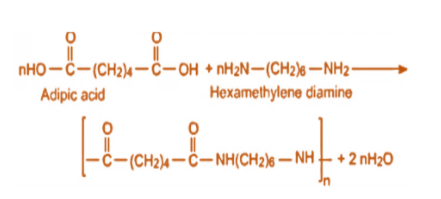
Hence, the monomer of Nylon 6 is \[NH{(C{H_2})_5}CO\] which we derive from caprolactam and of Nylon 6,6 is hexamethylenediamine and adipic acid.
Note: Nylons are basically described according to their numbers such as 6, 66, 11, 12, etc., which are related to their molecular structures. Although Nylon 6 and Nylon 6,6 are two different types, yet they have very similar mechanical, thermal and electrical properties. Both of them are available in different colours and are used for making sheets, rod or tubes.
Complete step-by-step answer:
We know that a monomer is a molecule that can be bonded to other identical molecules so as to form a polymer for polymeric chain. Nylon 6 and Nylon 6,6 are the two polymers made up of single unit monomers so that by repeating those units we can get these polymers.
Nylon 6 is also known as polycaprolactam. It is a polymer which is formed by ring-opening polymerization of caprolactam. So, we can say that the polymer of nylon 6 is g-caprolactam. During polymerization, an amide bond breaks within each caprolactam molecule. Therefore, the monomeric repeating unit of nylon 6 polymer is \[NH{(C{H_2})_5}CO\] which we derive from caprolactam

To prepare nylon 6,6, hexamethylenediamine and adipic acid are added under high pressure so that condensation polymerisation takes place. This clearly tells us that Nylon 6,6 contains amide bonds which are linking the monomeric units and therefore we can call it a polyamide. It is a type of step-growth polymerization, which means it takes place between monomers containing functional groups.
So, the monomers of Nylon 6,6 are hexamethylenediamine and adipic acid which contain functional groups such as amine and carboxylic acid which react in order to produce polymers containing different functionalities than their parent monomers.

Hence, the monomer of Nylon 6 is \[NH{(C{H_2})_5}CO\] which we derive from caprolactam and of Nylon 6,6 is hexamethylenediamine and adipic acid.
Note: Nylons are basically described according to their numbers such as 6, 66, 11, 12, etc., which are related to their molecular structures. Although Nylon 6 and Nylon 6,6 are two different types, yet they have very similar mechanical, thermal and electrical properties. Both of them are available in different colours and are used for making sheets, rod or tubes.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




