
What are the products obtained after ozonolysis of the following compounds: 2,4,4- trimethylpent-2-ene; 2-methylbut-1-ene; 1-methylcyclohexene?
Answer
528k+ views
Hint: Ozonolysis is the process when any compound (basically alkenes) reacts with ozone and followed by hydrolysis yields various compounds. This involves the cleavage of the alkene from the double bonds and then its workup to form aldehydes or ketones.
Complete answer:
Ozonolysis is the addition of the ozone molecule to the alkene. The addition of ozone, ${{O}_{3}}$to alkenes leads to the formation of an intermediate compound called ozonide. This ozonide has the oxygen atoms attached on the double bonds of the alkene. Ozonide undergoes cleavage in the presence of zinc and water to form ketones and aldehydes.
The given compounds on ozonolysis give:
-2,4,4 - trimethylpent-2-ene undergoes cleavage from the double bond to form 2,2 – dimethylpropanal and propanone. The reaction is:
${{(C{{H}_{3}})}_{3}}CH=C{{(C{{H}_{3}})}_{2}}\xrightarrow[Zn/{{H}_{2}}O]{{{O}_{3}}}{{(C{{H}_{3}})}_{3}}CH=O+O=C{{(C{{H}_{3}})}_{2}}$
-2-methylbut-1-ene undergoes ozonolysis to add oxygen on the double bonds and form butanone and methanal. The reaction is:
$C{{H}_{3}}C{{H}_{2}}C(C{{H}_{3}})=C{{H}_{2}}\xrightarrow[Zn/{{H}_{2}}O]{{{O}_{3}}}C{{H}_{3}}C{{H}_{2}}C(C{{H}_{3}})=O+O=C{{H}_{2}}$
-1-methylcyclohexene is a cyclic compound that undergoes cleavage from the double bond through ozonolysis and forms 6 – oxoheptanal. The reaction is:
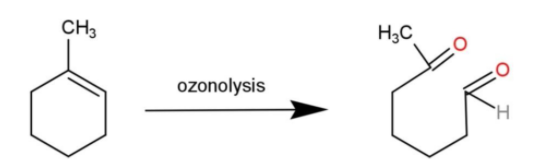
Hence, the product obtained in 2,4,4- trimethylpent-2-ene; 2-methylbut-1-ene; 1-methylcyclohexene are 2,2 – dimethylpropanal and propanone; butanone and methanol; 6 – oxoheptanal respectively.
Note:
Ozonolysis is used to detect the position of double bonds in any alkene. The reactions shown are the reductive workup reactions. The oxidative workup involves the reaction of ozonide with hydrogen peroxide. The oxidative workup will convert the aldehydes formed into their respective carboxylic acids, while ketones will remain the same.
Complete answer:
Ozonolysis is the addition of the ozone molecule to the alkene. The addition of ozone, ${{O}_{3}}$to alkenes leads to the formation of an intermediate compound called ozonide. This ozonide has the oxygen atoms attached on the double bonds of the alkene. Ozonide undergoes cleavage in the presence of zinc and water to form ketones and aldehydes.
The given compounds on ozonolysis give:
-2,4,4 - trimethylpent-2-ene undergoes cleavage from the double bond to form 2,2 – dimethylpropanal and propanone. The reaction is:
${{(C{{H}_{3}})}_{3}}CH=C{{(C{{H}_{3}})}_{2}}\xrightarrow[Zn/{{H}_{2}}O]{{{O}_{3}}}{{(C{{H}_{3}})}_{3}}CH=O+O=C{{(C{{H}_{3}})}_{2}}$
-2-methylbut-1-ene undergoes ozonolysis to add oxygen on the double bonds and form butanone and methanal. The reaction is:
$C{{H}_{3}}C{{H}_{2}}C(C{{H}_{3}})=C{{H}_{2}}\xrightarrow[Zn/{{H}_{2}}O]{{{O}_{3}}}C{{H}_{3}}C{{H}_{2}}C(C{{H}_{3}})=O+O=C{{H}_{2}}$
-1-methylcyclohexene is a cyclic compound that undergoes cleavage from the double bond through ozonolysis and forms 6 – oxoheptanal. The reaction is:

Hence, the product obtained in 2,4,4- trimethylpent-2-ene; 2-methylbut-1-ene; 1-methylcyclohexene are 2,2 – dimethylpropanal and propanone; butanone and methanol; 6 – oxoheptanal respectively.
Note:
Ozonolysis is used to detect the position of double bonds in any alkene. The reactions shown are the reductive workup reactions. The oxidative workup involves the reaction of ozonide with hydrogen peroxide. The oxidative workup will convert the aldehydes formed into their respective carboxylic acids, while ketones will remain the same.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




