
What are the structural isomers of ${C_5}{H_{12}}O$?
Answer
507.3k+ views
Hint: A structural isomer which is also known as a constitutional isomer is one in which two or more organic compounds consist of the same molecular formula but different structures. The compounds may differ in the location of substituent groups, functional groups and multiple bonds.
Complete answer:
To determine the isomers of any compound, first we need to calculate its degree of dissociation in order to check whether the given compound is saturated or unsaturated and the possibility of formation of a ring can also be determined with the help of degree of unsaturation.
Degree of unsaturation: In the analysis of molecular formula of organic molecules, the degree of unsaturation is a value which determines the total number of rings and number of pi bonds a compound may contain. It can be determined with the help of following formula:
Degree of unsaturation $ = C - \dfrac{H}{2} - \dfrac{X}{2} + \dfrac{N}{2} + 1$
Where, C is the number of carbon atoms, H is the number of hydrogen atoms, X is the number of halogen atoms and N is the number of nitrogen atoms present in the compound.
Now, for the given compound i.e., ${C_5}{H_{12}}O$ the value of degree of unsaturation will be as follows:
Degree of unsaturation $ = 5 - \dfrac{{12}}{2} + 1 \Rightarrow 0$
It means the molecule is an acyclic alkane and no multiple bonds are present within the structure.
Therefore, the possible structural isomers of the given compound are as follows:
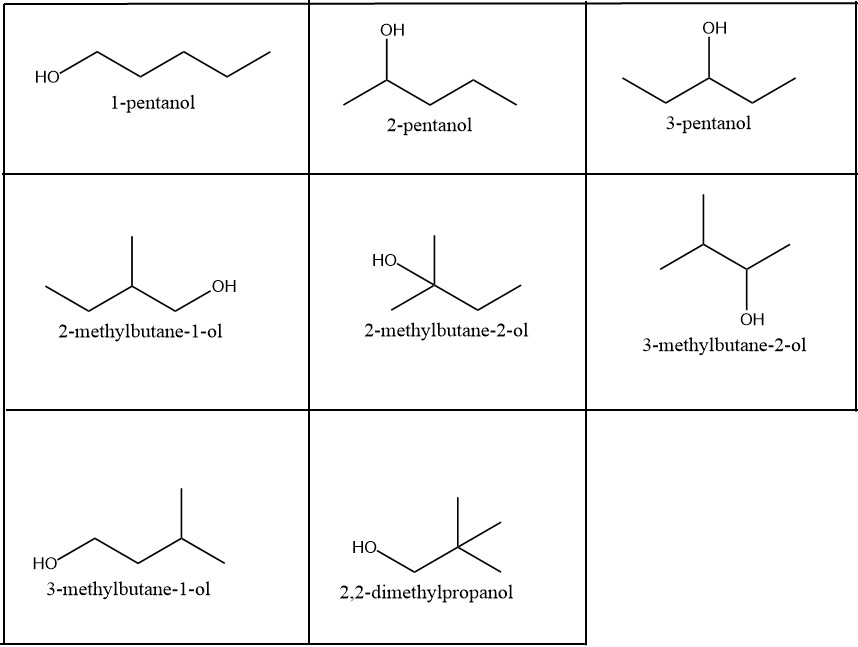
Eight alcohols structural isomers are possible for the given compound which are represented as:
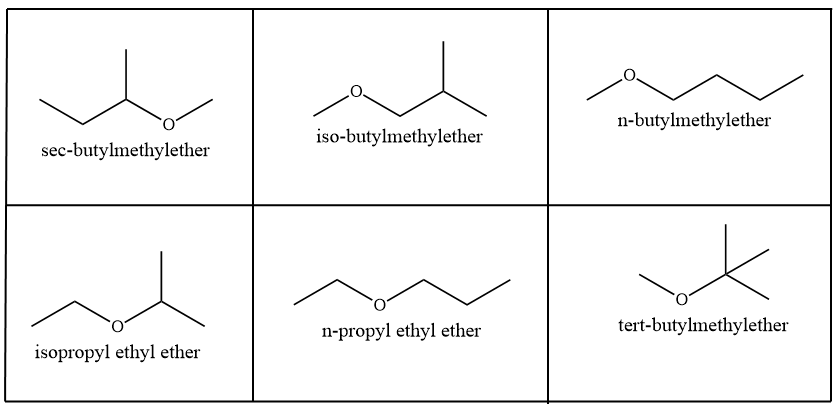
Six ether isomers are possible for the given compound which are represented as:
Note:
It is important to note that there is no specific formula to determine the structures or number of isomers possible for a compound. You have to try each combination of the atoms and arrange in every possible arrangement in order to determine the number of isomers possible.
Complete answer:
To determine the isomers of any compound, first we need to calculate its degree of dissociation in order to check whether the given compound is saturated or unsaturated and the possibility of formation of a ring can also be determined with the help of degree of unsaturation.
Degree of unsaturation: In the analysis of molecular formula of organic molecules, the degree of unsaturation is a value which determines the total number of rings and number of pi bonds a compound may contain. It can be determined with the help of following formula:
Degree of unsaturation $ = C - \dfrac{H}{2} - \dfrac{X}{2} + \dfrac{N}{2} + 1$
Where, C is the number of carbon atoms, H is the number of hydrogen atoms, X is the number of halogen atoms and N is the number of nitrogen atoms present in the compound.
Now, for the given compound i.e., ${C_5}{H_{12}}O$ the value of degree of unsaturation will be as follows:
Degree of unsaturation $ = 5 - \dfrac{{12}}{2} + 1 \Rightarrow 0$
It means the molecule is an acyclic alkane and no multiple bonds are present within the structure.
Therefore, the possible structural isomers of the given compound are as follows:
Eight alcohols structural isomers are possible for the given compound which are represented as:

Six ether isomers are possible for the given compound which are represented as:

Note:
It is important to note that there is no specific formula to determine the structures or number of isomers possible for a compound. You have to try each combination of the atoms and arrange in every possible arrangement in order to determine the number of isomers possible.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




