
What are the valencies of sulphur in $S{O_2}$ and $S{O_3}$?
Answer
522.9k+ views
Hint: We have to know that the sulphur is an element which lies in period 3 of the modern periodic table. The significance of sulphur is that it can have an extended valency. It’s valency ranges from -1 to +6. Hence, the maximum valency of sulphur is 6. Thus, this extended valency enables it to bond with different atom ions easily, be it a cation or an anion.
Complete step by step solution:
We have to remember that the sulphur has 6 electrons in the outermost shell. But it can have a valency of 6 as well and that of 2 as well.
Let’s look at $S{O_2}$ molecules. Here, one atom of sulphur is bonded with two atoms of oxygen.
We know that oxygen atoms have a valency of 2. It has 6 atoms in the outermost shell and it needs only two electrons from another atom in bonding to complete its octet. For two atoms of oxygen we need in total four electrons.
Therefore, in this case in $S{O_2}$ molecule the valency of sulphur is +4.
Now we can draw the structure of $S{O_2}$ as,
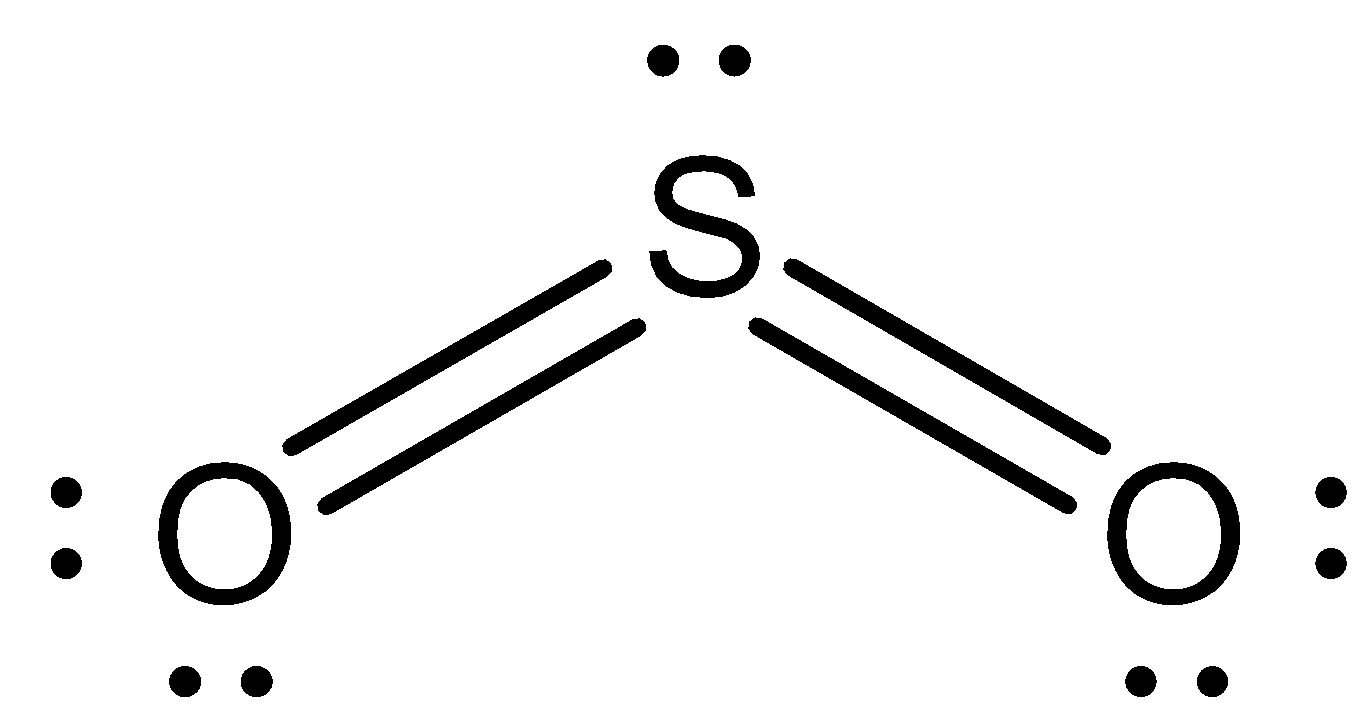
Now, in sulphur trioxide there are three oxygen atoms bonded with one sulphur atom.
The total valency of three oxygen atoms is -6. Hence in sulphur trioxide the valency of sulphur is +6 to satisfy the -6 valency of three oxygen atoms.
Now we can draw the structure of $S{O_3}$ as,
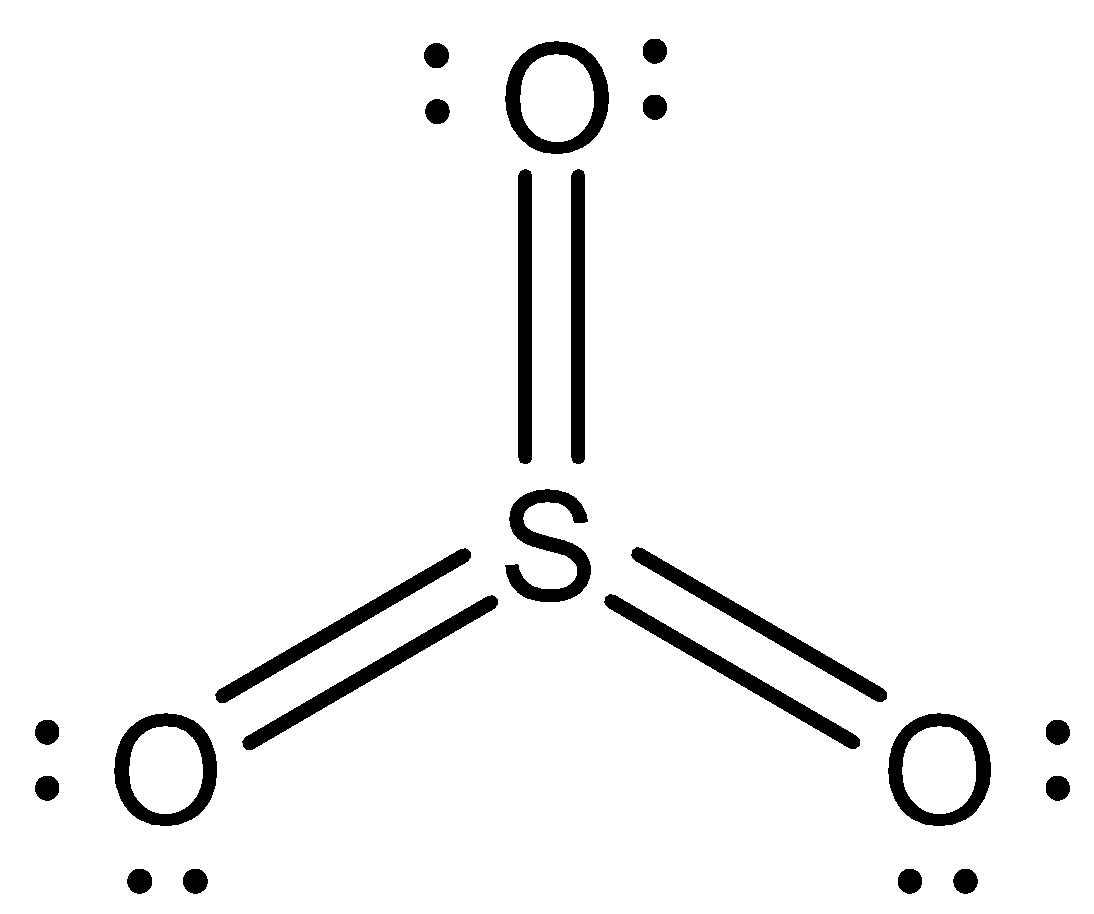
Note:We have to remember that elements such as sulphur and phosphorus have extended valencies. This is because their valence shell has enough orbitals to accommodate more electrons. If we talk about sulphur, it has vacant d-orbitals to accommodate electrons. That is the reason why in sulphur dioxide it shows +4 valency and in sulphur trioxide it shows +6 valency.
Complete step by step solution:
We have to remember that the sulphur has 6 electrons in the outermost shell. But it can have a valency of 6 as well and that of 2 as well.
Let’s look at $S{O_2}$ molecules. Here, one atom of sulphur is bonded with two atoms of oxygen.
We know that oxygen atoms have a valency of 2. It has 6 atoms in the outermost shell and it needs only two electrons from another atom in bonding to complete its octet. For two atoms of oxygen we need in total four electrons.
Therefore, in this case in $S{O_2}$ molecule the valency of sulphur is +4.
Now we can draw the structure of $S{O_2}$ as,

Now, in sulphur trioxide there are three oxygen atoms bonded with one sulphur atom.
The total valency of three oxygen atoms is -6. Hence in sulphur trioxide the valency of sulphur is +6 to satisfy the -6 valency of three oxygen atoms.
Now we can draw the structure of $S{O_3}$ as,

Note:We have to remember that elements such as sulphur and phosphorus have extended valencies. This is because their valence shell has enough orbitals to accommodate more electrons. If we talk about sulphur, it has vacant d-orbitals to accommodate electrons. That is the reason why in sulphur dioxide it shows +4 valency and in sulphur trioxide it shows +6 valency.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




