
What are Y and Z in the following reaction sequence?
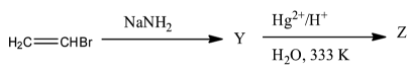
A. Y-ethyne, Z-acetic acid
B. Y-ethyne, Z-ethanal
C. Y-ethylene, Z-ethanal
D. Y-ethane, Z-ethanol

Answer
594.9k+ views
Hint: ${\rm{NaN}}{{\rm{H}}_{\rm{2}}}$dissociates into ${\rm{N}}{{\rm{a}}^ + }$ and ${\rm{N}}{{\rm{H}}_{\rm{2}}}^ - $. The ${\rm{N}}{{\rm{H}}_{\rm{2}}}^ - $ reacts with the alkyl halide giving the product Y. The product Y will help us to find Z accordingly.
Complete step-by-step answer:
The given reaction is,
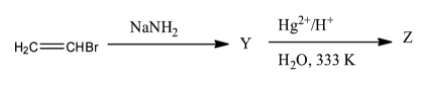
To find Y and Z we will consider the following stepwise reactions.
Step 1:
Sodium amide, ${\rm{NaN}}{{\rm{H}}_{\rm{2}}}$ dissociates into a sodium ion, ${\rm{N}}{{\rm{a}}^ + }$and ${\rm{N}}{{\rm{H}}_{\rm{2}}}^ - $.

Step 2:
The ${\rm{N}}{{\rm{H}}_{\rm{2}}}^ - $ ion then reacts with the ethyl bromide to generate the product Y. Product Y is an ethyne.
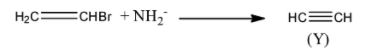
Step 3:
Now, this ethyne will react with ${\rm{H}}{{\rm{g}}^{{\rm{2 + }}}}{\rm{/}}{{\rm{H}}^{\rm{ + }}}$ and water at ${\rm{30}}{{\rm{0}}^{\rm{^\circ }}}{\rm{C}}$ generating the product Z.

The product Z is ethanal.
Alkyl halide is marked by the presence of a halide group with an alkyl group, that is presence of bromide with the ethene molecule gives the name to the compound ${{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{3}}}{\rm{Br}}$, ethyl bromide. Ethyne is marked by the presence of triple bonds between the two carbon atoms. Ethanol is marked by the presence of an aldehyde group. The conversion of the ethyne to the ethanal requires a temperature of ${\rm{30}}{{\rm{0}}^{\rm{^\circ }}}{\rm{C}}$ and in this reaction, water is used as the solvent.
So, out of the given options, B is the correct option, that is, Y-ethyne, Z-ethanal.
Note: Students may find it difficult to predict multiple products in a reaction. So, we should first start with the product that comes first. The finding of the first product will have to find the second product.
Complete step-by-step answer:
The given reaction is,

To find Y and Z we will consider the following stepwise reactions.
Step 1:
Sodium amide, ${\rm{NaN}}{{\rm{H}}_{\rm{2}}}$ dissociates into a sodium ion, ${\rm{N}}{{\rm{a}}^ + }$and ${\rm{N}}{{\rm{H}}_{\rm{2}}}^ - $.

Step 2:
The ${\rm{N}}{{\rm{H}}_{\rm{2}}}^ - $ ion then reacts with the ethyl bromide to generate the product Y. Product Y is an ethyne.
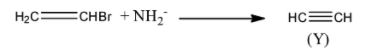
Step 3:
Now, this ethyne will react with ${\rm{H}}{{\rm{g}}^{{\rm{2 + }}}}{\rm{/}}{{\rm{H}}^{\rm{ + }}}$ and water at ${\rm{30}}{{\rm{0}}^{\rm{^\circ }}}{\rm{C}}$ generating the product Z.

The product Z is ethanal.
Alkyl halide is marked by the presence of a halide group with an alkyl group, that is presence of bromide with the ethene molecule gives the name to the compound ${{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{3}}}{\rm{Br}}$, ethyl bromide. Ethyne is marked by the presence of triple bonds between the two carbon atoms. Ethanol is marked by the presence of an aldehyde group. The conversion of the ethyne to the ethanal requires a temperature of ${\rm{30}}{{\rm{0}}^{\rm{^\circ }}}{\rm{C}}$ and in this reaction, water is used as the solvent.
So, out of the given options, B is the correct option, that is, Y-ethyne, Z-ethanal.
Note: Students may find it difficult to predict multiple products in a reaction. So, we should first start with the product that comes first. The finding of the first product will have to find the second product.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




