
Arrange in decreasing order of reactivity with HCl:
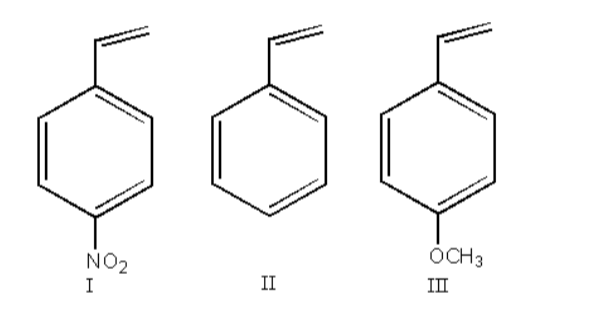
A. I < III < II
B. III > II > I
C. III > I > II
D. None of the above

Answer
573.9k+ views
Hint: The reactivity of reactants is decided on the basis of the stability of the intermediate. The more stable the intermediate, the more reactive the reactant. The substituents attached to the reactant affect the stability of the intermediate.
Complete Step by step answer: The reaction of an alkene with hydrochloric acid takes place in two steps. In the first step carbocation forms and in the second step product alkyl chloride forms. The reactivity of reactants is decided on the basis of the stability of the intermediate.
Nucleophilic attack of an alkene on the acid takes place so, alkene gets protonated and a carbocation form. On this carbocation the chloride ion attacks and forms alkyl chloride.
The reaction of each reactant is as follows:
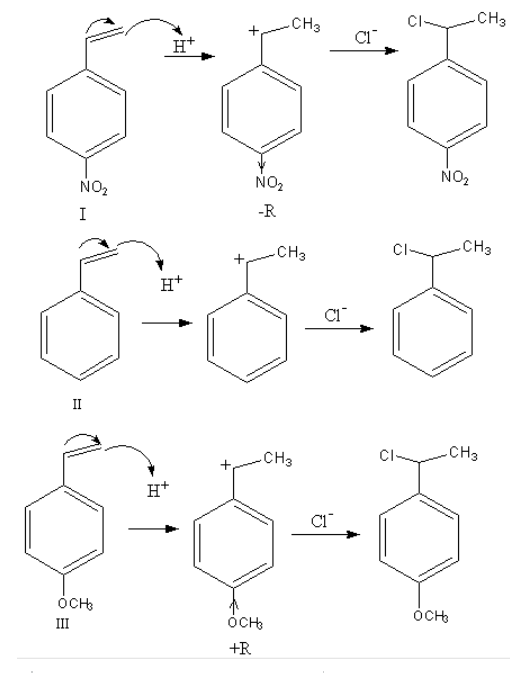
As during the reaction, a carbocation forms so, the rate of reaction depends upon the stability of the carbocation. The more stable the carbocation the more will be the rate of reaction.
The stability of carbocation depends upon the substituents attached to the benzene ring.
$ - {\text{OC}}{{\text{H}}_{}}$ is an electron-donating group so, it stabilizes the carbocation so, the reactivity of the reactant- III will be highest.
$ - {\text{N}}{{\text{O}}_2}$ is an electron-withdrawing group so, it destabilizes the carbocation so, the reactivity of the reactant- II will be lowest.
So, the decreasing order of reactivity with HCl is as follows:
III > II > I
Therefore, option (B) III > II > I, is correct.
Note: The ${{\text{S}}_{\text{N}}}1$ mechanism, a carbocation form in first step. The carbocation is the intermediate of ${{\text{S}}_{\text{N}}}1$types reactions. Carbocation is an electron-deficient species. So, the electron-donating group stabilizes the carbocation. Electron-withdrawing groups destabilize the carbocation. The more the number of electron-donating groups the more stable the carbocation will be. Here, all the carbocations are secondary. If all carbocations are different then the stability order of the carbocations, ${1^ \circ } < \,{2^ \circ }\, < \,{3^ \circ }$.
Complete Step by step answer: The reaction of an alkene with hydrochloric acid takes place in two steps. In the first step carbocation forms and in the second step product alkyl chloride forms. The reactivity of reactants is decided on the basis of the stability of the intermediate.
Nucleophilic attack of an alkene on the acid takes place so, alkene gets protonated and a carbocation form. On this carbocation the chloride ion attacks and forms alkyl chloride.
The reaction of each reactant is as follows:

As during the reaction, a carbocation forms so, the rate of reaction depends upon the stability of the carbocation. The more stable the carbocation the more will be the rate of reaction.
The stability of carbocation depends upon the substituents attached to the benzene ring.
$ - {\text{OC}}{{\text{H}}_{}}$ is an electron-donating group so, it stabilizes the carbocation so, the reactivity of the reactant- III will be highest.
$ - {\text{N}}{{\text{O}}_2}$ is an electron-withdrawing group so, it destabilizes the carbocation so, the reactivity of the reactant- II will be lowest.
So, the decreasing order of reactivity with HCl is as follows:
III > II > I
Therefore, option (B) III > II > I, is correct.
Note: The ${{\text{S}}_{\text{N}}}1$ mechanism, a carbocation form in first step. The carbocation is the intermediate of ${{\text{S}}_{\text{N}}}1$types reactions. Carbocation is an electron-deficient species. So, the electron-donating group stabilizes the carbocation. Electron-withdrawing groups destabilize the carbocation. The more the number of electron-donating groups the more stable the carbocation will be. Here, all the carbocations are secondary. If all carbocations are different then the stability order of the carbocations, ${1^ \circ } < \,{2^ \circ }\, < \,{3^ \circ }$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




