
Arrange the following compounds in the order of increasing boiling points: 1-Chloropropane, Isopropyl chloride, 1-Chlorobutane.
Answer
585k+ views
Hint: In case of alkyl halides that have the same halogen, group attached, the boiling point is directly proportional to the size of the alkyl group and inversely proportional to the branching in the chain.
Complete step by step answer:
Boiling point is that temperature at which the vapour pressure of the compound becomes equal to the atmospheric pressure i.e. 1 atm. We can also define it as the temperature at which a compound in its liquid phase gets transformed into a gaseous phase. Putting it more accurately, boiling point is the temperature at which there is an equilibrium between the gaseous and liquid phase of a compound.
The intermolecular forces between molecules are broken at the boiling point and the molecules are isolated and packed loosely so that they get converted into the phase of gas. The energy that is needed to break the intermolecular forces is catered by increasing the temperature.
As the molecular weight increases the boiling point also increases because more energy is needed to break the intermolecular forces of interaction, like dipole-dipole interaction, Vander Waals forces, etc. present in the compound.
In case of isomers, boiling point is higher for compounds having straight chains. With the increase in the molecular branching, there is a decrease in the intermolecular forces between the molecules because of steric effect and thus, lower energy is needed to break those interactions and there is a decrease in boiling point.
Vapour pressure is high in case of branched chains because of less intermolecular forces present in the compound and therefore, boiling point is lower.
Now, look at all three compounds provided, 1-chlorobutane has more weight due to an extra carbon. Therefore, it has the highest boiling point compared to both. Whereas isopropyl alcohol has branching in it, so it has the lowest boiling point.
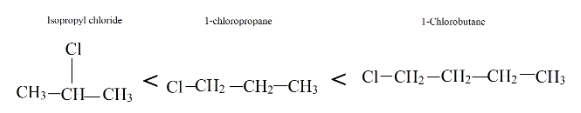
Therefore, the order of increasing boiling point is: Isopropyl chloride < 1-chloropropane < 1-chlorobutane.
Note:
The relation between vapour pressure and boiling point must be noted. With an increase in the vapour pressure of the compounds, boiling point decreases. And, with an increase in the number of carbon atoms, boiling point increases because more energy is needed to separate them as they have Vander Waal attractive forces in them.
Complete step by step answer:
Boiling point is that temperature at which the vapour pressure of the compound becomes equal to the atmospheric pressure i.e. 1 atm. We can also define it as the temperature at which a compound in its liquid phase gets transformed into a gaseous phase. Putting it more accurately, boiling point is the temperature at which there is an equilibrium between the gaseous and liquid phase of a compound.
The intermolecular forces between molecules are broken at the boiling point and the molecules are isolated and packed loosely so that they get converted into the phase of gas. The energy that is needed to break the intermolecular forces is catered by increasing the temperature.
As the molecular weight increases the boiling point also increases because more energy is needed to break the intermolecular forces of interaction, like dipole-dipole interaction, Vander Waals forces, etc. present in the compound.
In case of isomers, boiling point is higher for compounds having straight chains. With the increase in the molecular branching, there is a decrease in the intermolecular forces between the molecules because of steric effect and thus, lower energy is needed to break those interactions and there is a decrease in boiling point.
Vapour pressure is high in case of branched chains because of less intermolecular forces present in the compound and therefore, boiling point is lower.
Now, look at all three compounds provided, 1-chlorobutane has more weight due to an extra carbon. Therefore, it has the highest boiling point compared to both. Whereas isopropyl alcohol has branching in it, so it has the lowest boiling point.
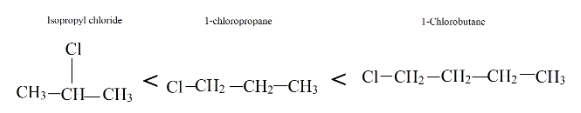
Therefore, the order of increasing boiling point is: Isopropyl chloride < 1-chloropropane < 1-chlorobutane.
Note:
The relation between vapour pressure and boiling point must be noted. With an increase in the vapour pressure of the compounds, boiling point decreases. And, with an increase in the number of carbon atoms, boiling point increases because more energy is needed to separate them as they have Vander Waal attractive forces in them.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




