
Arrange the following in the increasing order of their basic strength.
$\text{C}{{\text{H}}_{3}}\text{N}{{\text{H}}_{2}}\text{ , }{{\left( \text{C}{{\text{H}}_{3}} \right)}_{2}}\text{NH , }{{\text{C}}_{6}}{{\text{H}}_{5}}\text{N}{{\text{H}}_{2}}\text{ , }{{\text{C}}_{6}}{{\text{H}}_{5}}\text{C}{{\text{H}}_{2}}\text{N}{{\text{H}}_{2}}$
Answer
588.9k+ views
Hint: Basic strength means the ability to donate electrons to electrophiles (electron deficient species). Check the factors on which the basicity depends and their effect. Here, $\text{C}{{\text{H}}_{3}}\text{N}{{\text{H}}_{2}}$ and ${{\left( \text{C}{{\text{H}}_{3}} \right)}_{2}}\text{NH}$ is aliphatic amines and ${{\text{C}}_{6}}{{\text{H}}_{5}}\text{N}{{\text{H}}_{2}}$ and ${{\text{C}}_{6}}{{\text{H}}_{5}}\text{C}{{\text{H}}_{2}}\text{N}{{\text{H}}_{2}}$ are aromatic amines.
Complete step by step answer:
Let us discuss the factors on which basic strength depends to get the general order of the bases:
A. Methyl amine: It has one methyl group attached to it and is aliphatic in nature. It is basic in nature.
B. Dimethyl amine: It has two methyl groups attached to it and is aliphatic in nature. It is highly basic.
C. Aniline: It has no methyl group attached. It is an aromatic amine in which $-\text{N}{{\text{H}}_{2}}$ group is attached to a benzene ring. The lone pair of $-\text{N}{{\text{H}}_{2}}$ group undergoes resonance with the benzene ring making it less basic.
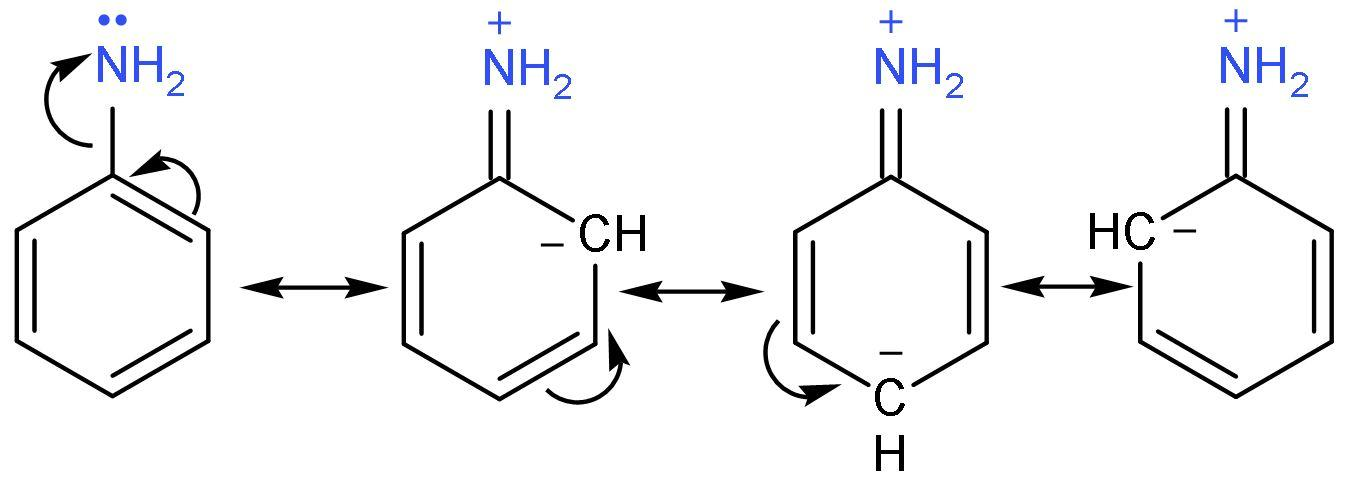
D. Benzyl amine: It is primary amine and lone pair of nitrogen does not undergo resonance with benzene ring due to $\left( -\text{C}{{\text{H}}_{2}}- \right)$ group in between the two. The $-\text{I}$ effect of phenyl group decreases the basicity.
The increasing order of their basic strength is \[{{\left( \text{C}{{\text{H}}_{3}} \right)}_{2}}\text{NH}>\text{C}{{\text{H}}_{3}}\text{N}{{\text{H}}_{2}}>{{\text{C}}_{6}}{{\text{H}}_{5}}\text{C}{{\text{H}}_{2}}\text{N}{{\text{H}}_{2}}>{{\text{C}}_{6}}{{\text{H}}_{5}}\text{N}{{\text{H}}_{2}}\].
Note: In aqueous solution, the order of basic strength is \[{{\left( \text{C}{{\text{H}}_{3}} \right)}_{2}}\text{NH}>\text{C}{{\text{H}}_{3}}\text{N}{{\text{H}}_{2}}>{{\left( \text{C}{{\text{H}}_{3}} \right)}_{3}}\text{N}\]. The order mainly changes due to solvation effect (interaction between solute and solvent, here cations), inductive effect and steric hindrance (crowd of groups).
Complete step by step answer:
Let us discuss the factors on which basic strength depends to get the general order of the bases:
| S. No. | Factors of basicity | Definitions | Effect on basic strength | Reason of that effect |
| 1. | Inductive effect | It is displacement of sigma electrons due to electronegativity or electropositivity of elements. | Positive inductive effect increases the basicity and Negative inductive effect decreases the basicity | More the positive inductive effect (donation of electrons) or $+\text{I}$ effect which increases the electron density on the molecule. Due to which electron donating ability increases of a molecule, making it basic. Number of $+\text{I}$ groups on a molecule increases, its basicity increases. Whereas, $-\text{I}$ effect decreases the electron density on the molecule making it electron deficient reducing the basicity. Number of $-\text{I}$ groups attached increases, its basic strength decreases. Hence, order of basicity is tertiary$>$ secondary$>$ primary. As methyl groups attached produce $+\text{I}$effect. |
| 2. | Resonance | Resonance is actually the delocalization or movement of $\pi $ electrons. | Decreases the basicity | The delocalization of $\pi $ electrons makes it difficult for nitrogen to share its lone pair. Thus, the basic strength of compound decreases. Hence, aromatic amines are less basic than aliphatic amines. |
A. Methyl amine: It has one methyl group attached to it and is aliphatic in nature. It is basic in nature.
B. Dimethyl amine: It has two methyl groups attached to it and is aliphatic in nature. It is highly basic.
C. Aniline: It has no methyl group attached. It is an aromatic amine in which $-\text{N}{{\text{H}}_{2}}$ group is attached to a benzene ring. The lone pair of $-\text{N}{{\text{H}}_{2}}$ group undergoes resonance with the benzene ring making it less basic.

D. Benzyl amine: It is primary amine and lone pair of nitrogen does not undergo resonance with benzene ring due to $\left( -\text{C}{{\text{H}}_{2}}- \right)$ group in between the two. The $-\text{I}$ effect of phenyl group decreases the basicity.
The increasing order of their basic strength is \[{{\left( \text{C}{{\text{H}}_{3}} \right)}_{2}}\text{NH}>\text{C}{{\text{H}}_{3}}\text{N}{{\text{H}}_{2}}>{{\text{C}}_{6}}{{\text{H}}_{5}}\text{C}{{\text{H}}_{2}}\text{N}{{\text{H}}_{2}}>{{\text{C}}_{6}}{{\text{H}}_{5}}\text{N}{{\text{H}}_{2}}\].
Note: In aqueous solution, the order of basic strength is \[{{\left( \text{C}{{\text{H}}_{3}} \right)}_{2}}\text{NH}>\text{C}{{\text{H}}_{3}}\text{N}{{\text{H}}_{2}}>{{\left( \text{C}{{\text{H}}_{3}} \right)}_{3}}\text{N}\]. The order mainly changes due to solvation effect (interaction between solute and solvent, here cations), inductive effect and steric hindrance (crowd of groups).
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




