
Arrange the following nucleophiles in the decreasing order of nucleophilicity:
A.
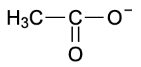
B. $CH_{3}O^{-}$
C. $CN^{-}$
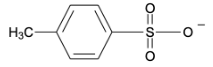
D.
I. C, B, A, D
II. A, B, C, D
III. D, C, B, A
IV. D, A, C, B


Answer
541.8k+ views
Hint:The nucleophilicity of different nucleophiles depends on many things. And one amongst them is increasing base strength indicates decreasing nucleophilic strength.
Complete step-by-step answer:- Nucleophilicity of the nucleophile strength is referring to a substance’s nucleophilic character and is often used to compare the affinity of atoms.
- It describes the affinity of a nucleophile to the positively charged atomic nuclei of atoms.
- We know that nucleophiles are electron rich species in search of nucleus or positive center (Nucleus – nucleus, phile – loving). Therefore, these can be either negatively charged or even neutral.
- Now, in order to solve the question, we need to consider the conjugate bases of the given options and then obtain the order of acidic nature.
- The conjugate bases are shown below:
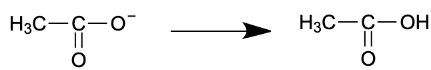
$CH_{3}O^{-}\rightarrow CH_{3}OH$
$CN^{-}\rightarrow HCN$
$p-H_{3}C-C_{6}H_{4}-\ SO_{2}-O^{-}\rightarrow p-H_{3}C-C_{6}H_{4}-SO_{2}-OH$
- The order of acidic nature is D > A > C > B
- So, the increasing order of nucleophilicity will be B > C > A > D
Therefore, the correct answer is (D).
Note:The important thing to keep in mind is that the order of nucleophilicity is the reverse: the order of basicity and nucleophilicity increases down the group in the periodic table. Lesser the electronegativity of donor atoms more is its tendency to give a lone pair of electrons, and thus more is its nucleophilic strength.
Complete step-by-step answer:- Nucleophilicity of the nucleophile strength is referring to a substance’s nucleophilic character and is often used to compare the affinity of atoms.
- It describes the affinity of a nucleophile to the positively charged atomic nuclei of atoms.
- We know that nucleophiles are electron rich species in search of nucleus or positive center (Nucleus – nucleus, phile – loving). Therefore, these can be either negatively charged or even neutral.
- Now, in order to solve the question, we need to consider the conjugate bases of the given options and then obtain the order of acidic nature.
- The conjugate bases are shown below:

$CH_{3}O^{-}\rightarrow CH_{3}OH$
$CN^{-}\rightarrow HCN$
$p-H_{3}C-C_{6}H_{4}-\ SO_{2}-O^{-}\rightarrow p-H_{3}C-C_{6}H_{4}-SO_{2}-OH$
- The order of acidic nature is D > A > C > B
- So, the increasing order of nucleophilicity will be B > C > A > D
Therefore, the correct answer is (D).
Note:The important thing to keep in mind is that the order of nucleophilicity is the reverse: the order of basicity and nucleophilicity increases down the group in the periodic table. Lesser the electronegativity of donor atoms more is its tendency to give a lone pair of electrons, and thus more is its nucleophilic strength.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




