
Aryl halides are less reactive in nucleophilic substitution reactions.
(i) write any two reasons for less reactivity
(ii) Give one example for nucleophilic substitution reaction of aryl halides
Answer
573.6k+ views
Hint:Nucleophiles are molecules which are nucleus loving and are attracted towards electron deficient species. Hence, if a molecule is more reactive in nucleophilic reactions they have a rich electron centre and if they are not reactive or less reactive, they have a weaker electron centre.
Complete step by step answer:
In organic chemistry, aryl means aromatic and halide means halogens. When we look for the aryl halide, it is simply one halogen atom bonded directly to a benzene ring. They are different from haloalkanes because they exhibit many differences in methods of preparation and properties. Aryl halides have a halogen directly bonded to carbon of an aromatic ring. Examples are bromobenzene, fluorobenzene etc.
Aryl halide are the compound in which halogen atom is attached to $s{p^2}$ hybridized carbon atom of benzene ring whereas in alkyl halides halogen is an attached to $s{p^2}$ hybridized carbon atom .
Reactivity of nucleophilic substitution reaction depends upon several factors like nature of leaving group, bond dissociation enthalpy of $C-X$ bond, solvent used.
The reasons for less reactivity are:
1. In aryl halide The carbon is $s{p^2}$ hybridised. In such carbons the s character is more, s character is synonymous with electronegativity. More the s character, more is the electronegative nature of that carbon. Hence, if a carbon is more electronegative, it pulls the electrons closer towards itself, making a reaction difficult.
2. Aromatic compounds have a rich electron centre in their ring due to delocalisation of π electrons, hence any incoming nucleophile will face repulsion.
Example of nucleophilic substitution reaction of aryl halides,
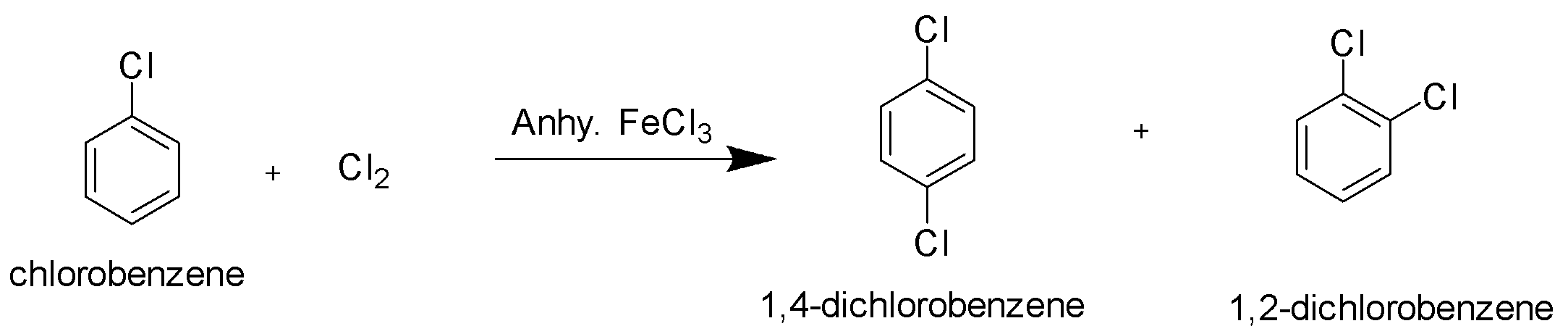
Halogenation of Chlorobenzene with chlorine gas in the presence of anhydrous ferric chloride gives disubstituted chloro products.
Hence option B is the correct option.
Note:
-If the substituents on the ring are of electron withdrawing nature, then the rate of nucleophilic reaction increases since they reduce the electron density on the rind, making it more reactive . These groups are electronegative groups such as carbonyl groups.
-Electron donating groups decrease the rate of reaction since they increase these groups increase the electron density on the ring. These groups are alkyl groups and halides due to positive inductive effect.
Complete step by step answer:
In organic chemistry, aryl means aromatic and halide means halogens. When we look for the aryl halide, it is simply one halogen atom bonded directly to a benzene ring. They are different from haloalkanes because they exhibit many differences in methods of preparation and properties. Aryl halides have a halogen directly bonded to carbon of an aromatic ring. Examples are bromobenzene, fluorobenzene etc.

Aryl halide are the compound in which halogen atom is attached to $s{p^2}$ hybridized carbon atom of benzene ring whereas in alkyl halides halogen is an attached to $s{p^2}$ hybridized carbon atom .
Reactivity of nucleophilic substitution reaction depends upon several factors like nature of leaving group, bond dissociation enthalpy of $C-X$ bond, solvent used.
The reasons for less reactivity are:
1. In aryl halide The carbon is $s{p^2}$ hybridised. In such carbons the s character is more, s character is synonymous with electronegativity. More the s character, more is the electronegative nature of that carbon. Hence, if a carbon is more electronegative, it pulls the electrons closer towards itself, making a reaction difficult.
2. Aromatic compounds have a rich electron centre in their ring due to delocalisation of π electrons, hence any incoming nucleophile will face repulsion.
Example of nucleophilic substitution reaction of aryl halides,
Halogenation of Chlorobenzene with chlorine gas in the presence of anhydrous ferric chloride gives disubstituted chloro products.

Hence option B is the correct option.
Note:
-If the substituents on the ring are of electron withdrawing nature, then the rate of nucleophilic reaction increases since they reduce the electron density on the rind, making it more reactive . These groups are electronegative groups such as carbonyl groups.
-Electron donating groups decrease the rate of reaction since they increase these groups increase the electron density on the ring. These groups are alkyl groups and halides due to positive inductive effect.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




