
Assertion (A): ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{CHO}}$ on heating with dilute NaOH forms aldol condensation product.
Reason(R): ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{CHO}}$ has no alpha-hydrogen.
A.Both A and R are true and R is the correct explanation of A
B.Both A and R are true and R is not the correct explanation of A.
C.A is true but R is false.
D.A is false but R is true.
Answer
582.3k+ views
Hint: Aldol condensation is an organic reaction in which enolate ion reacts with a carbonyl compound to form beta-hydroxy ketone or beta-hydroxy aldehyde. This is then followed by a dehydration process to give a conjugated enone. Aldol condensation is an important organic synthesis, to form a new carbon-carbon bond.
Complete step by step answer:
Mechanism for Aldol condensation reaction:
${\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\xrightarrow[{{{\text{H}}_{\text{2}}}{{O,\Delta }}}]{{{\text{NaOH}}}}{\text{C}}{{\text{H}}_{\text{3}}}{\text{CH = CHCHO}}$
Step 1: Deprotonation of aldehyde by hydroxide ion
Step 2: The enolate ion formed in step 1 will add to the unreacted aldehyde.
Step 3: The alkoxide ion formed in step 2 will be protonated by water and will form Aldol.
Step 4: A small amount of Aldol is converted into enolate ion by the hydroxide ion.
Step 5: The enolate ion formed in the above step will lose a hydroxide ion and will form alpha-beta-unsaturated aldehyde.
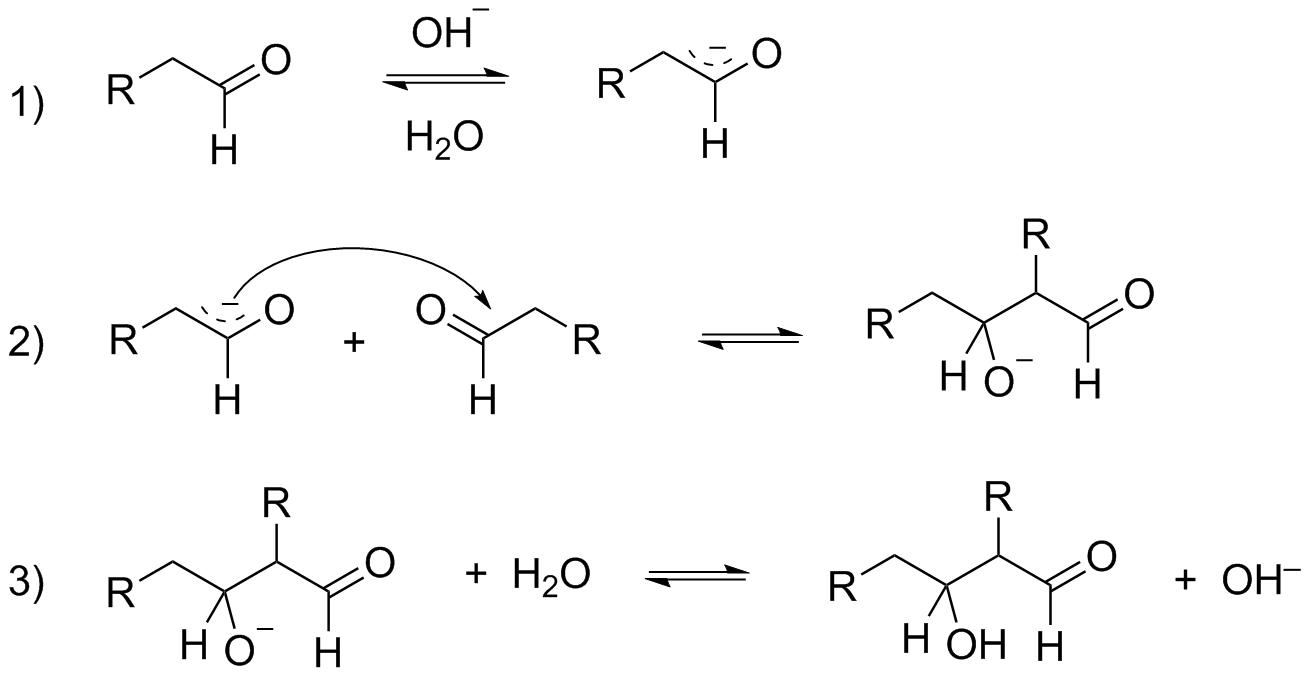
As you can notice in the mechanism of Aldol condensation presence of alpha-hydrogen is compulsory for the reaction to proceed. And when you look at ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{CHO}}$, there is no alpha-hydrogen and hence, the given compound will not undergo Aldol condensation.
Hence the assertion (A) is incorrect but the reason (R) is correct.
So, the correct answer is Option D .
Note:
Alpha-hydrogens are the hydrogens attached to the carbon atom next to the functional group. Now, as you have seen the presence of alpha-hydrogen is necessary for Aldol condensation. Compounds not having alpha-hydrogens undergo Clasien condensation reaction rather than Aldol condensation. Therefore, ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{CHO}}$ will undergo a Claisen condensation reaction.
Complete step by step answer:
Mechanism for Aldol condensation reaction:
${\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\xrightarrow[{{{\text{H}}_{\text{2}}}{{O,\Delta }}}]{{{\text{NaOH}}}}{\text{C}}{{\text{H}}_{\text{3}}}{\text{CH = CHCHO}}$
Step 1: Deprotonation of aldehyde by hydroxide ion
Step 2: The enolate ion formed in step 1 will add to the unreacted aldehyde.
Step 3: The alkoxide ion formed in step 2 will be protonated by water and will form Aldol.
Step 4: A small amount of Aldol is converted into enolate ion by the hydroxide ion.
Step 5: The enolate ion formed in the above step will lose a hydroxide ion and will form alpha-beta-unsaturated aldehyde.

As you can notice in the mechanism of Aldol condensation presence of alpha-hydrogen is compulsory for the reaction to proceed. And when you look at ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{CHO}}$, there is no alpha-hydrogen and hence, the given compound will not undergo Aldol condensation.
Hence the assertion (A) is incorrect but the reason (R) is correct.
So, the correct answer is Option D .
Note:
Alpha-hydrogens are the hydrogens attached to the carbon atom next to the functional group. Now, as you have seen the presence of alpha-hydrogen is necessary for Aldol condensation. Compounds not having alpha-hydrogens undergo Clasien condensation reaction rather than Aldol condensation. Therefore, ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{CHO}}$ will undergo a Claisen condensation reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




