
Assertion: Square planar complexes do not show optical isomerism.
Reason: Optical isomerism is due to the absence of elements of symmetry.
A. Both assertion and reason are correct and reason is the correct explanation for assertion.
B. Both assertion and reason are correct but reason is not the correct explanation for assertion.
C. Assertion is correct but the reason is incorrect.
D. Both assertion and reason are incorrect.
Answer
513k+ views
Hint: Optical isomerism: The compounds which have the same molecular and structural formula but cannot be superimposed on each other are known as optical isomers. The phenomenon is known as optical isomerism. In simple words, these compounds are non-superimposable mirror images of each other i.e. There must be no element of symmetry in a compound to show the optical isomerism.
Complete answer: In square planar complexes, a central atom is surrounded by four constituent atoms form corners of the square in the same plane. The coordination number of the central atom for the square planar complex is $4$.
Different types of square planar complexes are as follows:
$[M{A_4}]$: The structure is represented as follows:
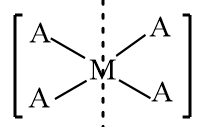
As in the given structure, all four constituent groups connected to the central atom are the same, so it is an achiral molecule and the structure consists of a plane of symmetry, therefore it is an optically inactive compound.
$[M{A_3}B]$: The structure is represented as follows:
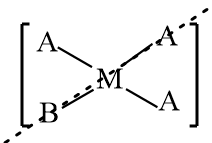
As in the given structure, three constituent groups connected to the central atom are the same, so it is an achiral molecule and the structure consists of a plane of symmetry, therefore it is an optically inactive compound.
$[M{A_2}{B_2}]$: The structure is represented as follows:
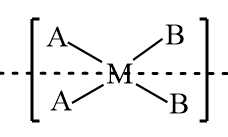
As in the given structure, two constituent groups connected to the central atom are the same, so it is an achiral molecule and the structure consists of a plane of symmetry, therefore it is an optically inactive compound.
$[MABCD]$: The structure is represented as follows:
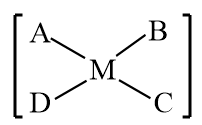
Although the molecule consists of four different constituent groups, but a molecule is achiral when a rotation which is a combination of a rotation and a reflection in a plane, results in the formation of the same molecule, due to which the compound or molecule will be optically inactive. Therefore, it is also an optically inactive molecule.
Hence, Square planar complexes do not show optical isomerism because optical isomerism in structures is due to the absence of elements of symmetry but all the structures of square planar complexes possess the element of symmetry.
Therefore, option (A) is the correct answer.
Note:
It is important to note that as all the constituent groups in the square planar complex are present in the same plane, therefore these complexes can show geometrical isomerism i.e., the arrangement of groups can be compared for these molecules. Total five geometrical isomers are possible for a square planar complex.
Complete answer: In square planar complexes, a central atom is surrounded by four constituent atoms form corners of the square in the same plane. The coordination number of the central atom for the square planar complex is $4$.
Different types of square planar complexes are as follows:
$[M{A_4}]$: The structure is represented as follows:

As in the given structure, all four constituent groups connected to the central atom are the same, so it is an achiral molecule and the structure consists of a plane of symmetry, therefore it is an optically inactive compound.
$[M{A_3}B]$: The structure is represented as follows:

As in the given structure, three constituent groups connected to the central atom are the same, so it is an achiral molecule and the structure consists of a plane of symmetry, therefore it is an optically inactive compound.
$[M{A_2}{B_2}]$: The structure is represented as follows:

As in the given structure, two constituent groups connected to the central atom are the same, so it is an achiral molecule and the structure consists of a plane of symmetry, therefore it is an optically inactive compound.
$[MABCD]$: The structure is represented as follows:

Although the molecule consists of four different constituent groups, but a molecule is achiral when a rotation which is a combination of a rotation and a reflection in a plane, results in the formation of the same molecule, due to which the compound or molecule will be optically inactive. Therefore, it is also an optically inactive molecule.
Hence, Square planar complexes do not show optical isomerism because optical isomerism in structures is due to the absence of elements of symmetry but all the structures of square planar complexes possess the element of symmetry.
Therefore, option (A) is the correct answer.
Note:
It is important to note that as all the constituent groups in the square planar complex are present in the same plane, therefore these complexes can show geometrical isomerism i.e., the arrangement of groups can be compared for these molecules. Total five geometrical isomers are possible for a square planar complex.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




