
Assertion: The nitration of chlorobenzene leads to the formation of m-nitrochlorobenzene.
Reason: $N{{O}_{2}}$ group is an m-directing group.
(a)- Both assertion and reason are correct and the reason is the correct explanation for the assertion.
(b)- Both assertion and reason are correct and the reason is not the correct explanation for the assertion.
(c)- Assertion is correct but the reason is incorrect.
(d)- Assertion is incorrect but the reason is correct.
Answer
585.9k+ views
Hint: At meta-position, the halogen gets attached when the halogen is reacted with nitrobenzene by Electrophilic substitution reaction. Due to the presence of $N{{O}_{2}}$ on the benzene ring, the incoming electrophile attacks the meta position of the benzene ring.
Complete step by step answer:
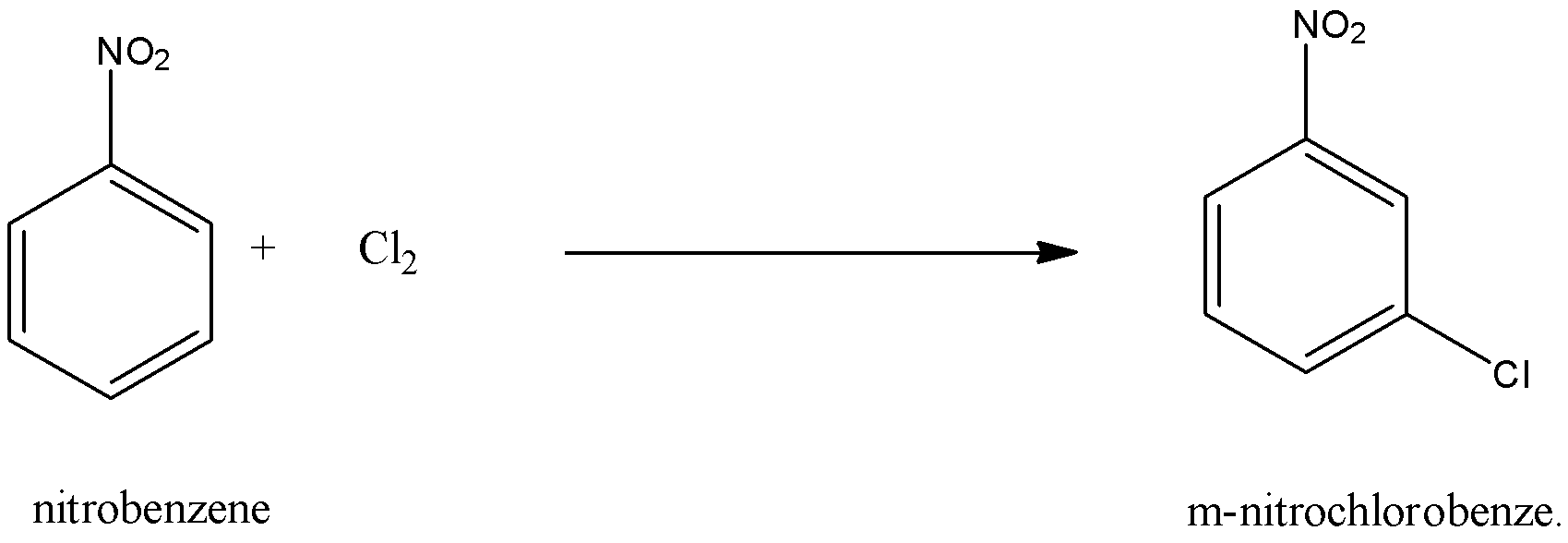
The assertion is not true, because not the nitration of chlorobenzene leads to the formation of m-nitrochlorobenzene but the chlorination of nitrobenzene leads to the formation of m-nitrochlorobenzene. The reason is true that the $N{{O}_{2}}$ group is an m-directing group.
This is explained below:
Nitrobenzene undergoes Electrophilic substitution reactions like halogenations, nitration, and sulfonation). Since the $N{{O}_{2}}$ group is a strongly deactivating and meta-directing group, therefore the incoming group enters the m-position. This may be explained as follows:
The resonance hybrid structures of Nitrobenzene are as follows:
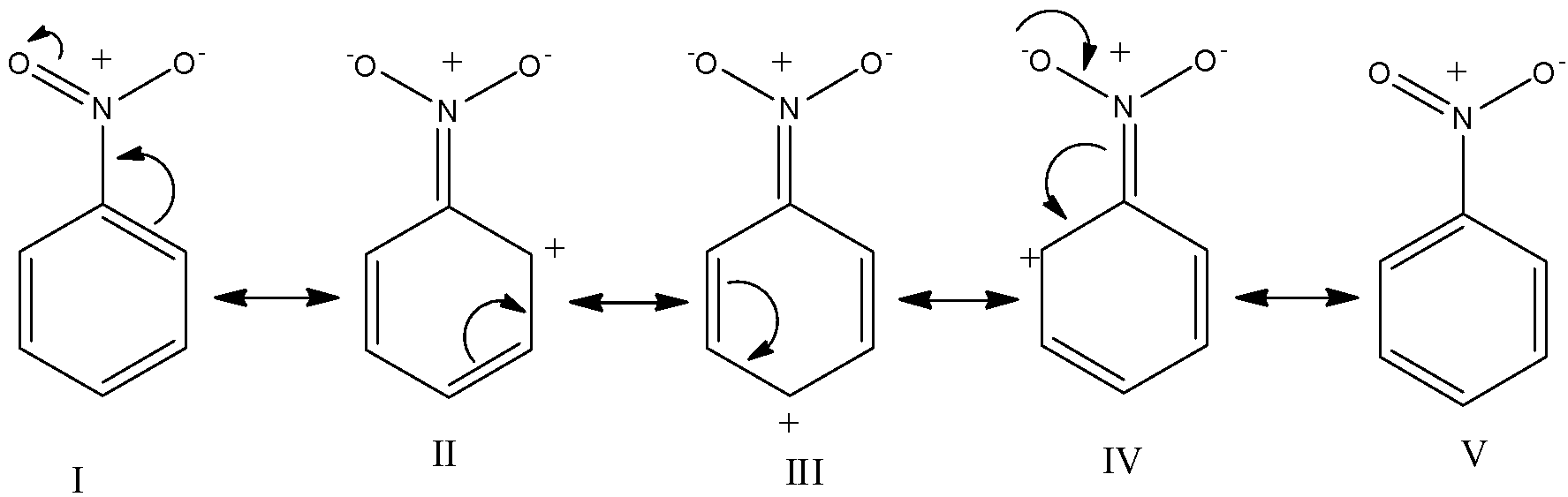
It is evident from the structure (II-IV) that the nitro group because of its electron-withdrawing nature reduces electron density more at ortho and para position than at meta position. In other words, the electron density is comparatively more at meta-position than at ortho or para position. Therefore, the nitro group is m-directing. Further since the $N{{O}_{2}}$ group is strongly deactivating the reaction occurs as follows:
So, the correct answer is an option (d)- Assertion is incorrect but the reason is correct.
Note: Nitro groups have electron-withdrawing nature due to –R-effect and –I-effect. The displacement of $\sigma -electrons$ along the saturated carbon chain whenever an electron-withdrawing or electron-donating group is present at the end of the carbon chain is called inductive effect or I-effect. If we see that the substituent which is attached to the end of the carbon chain is electron-withdrawing, the effect is called –I-Effect. The flow of electrons from one part of the conjugated system to the other creating centers of low and high electron density due to the phenomenon of resonance is called resonance effect or R-effect. Groups that withdraw electrons from the double bond are said to have –R-effect.
Complete step by step answer:
The assertion is not true, because not the nitration of chlorobenzene leads to the formation of m-nitrochlorobenzene but the chlorination of nitrobenzene leads to the formation of m-nitrochlorobenzene. The reason is true that the $N{{O}_{2}}$ group is an m-directing group.
This is explained below:
Nitrobenzene undergoes Electrophilic substitution reactions like halogenations, nitration, and sulfonation). Since the $N{{O}_{2}}$ group is a strongly deactivating and meta-directing group, therefore the incoming group enters the m-position. This may be explained as follows:
The resonance hybrid structures of Nitrobenzene are as follows:

It is evident from the structure (II-IV) that the nitro group because of its electron-withdrawing nature reduces electron density more at ortho and para position than at meta position. In other words, the electron density is comparatively more at meta-position than at ortho or para position. Therefore, the nitro group is m-directing. Further since the $N{{O}_{2}}$ group is strongly deactivating the reaction occurs as follows:

So, the correct answer is an option (d)- Assertion is incorrect but the reason is correct.
Note: Nitro groups have electron-withdrawing nature due to –R-effect and –I-effect. The displacement of $\sigma -electrons$ along the saturated carbon chain whenever an electron-withdrawing or electron-donating group is present at the end of the carbon chain is called inductive effect or I-effect. If we see that the substituent which is attached to the end of the carbon chain is electron-withdrawing, the effect is called –I-Effect. The flow of electrons from one part of the conjugated system to the other creating centers of low and high electron density due to the phenomenon of resonance is called resonance effect or R-effect. Groups that withdraw electrons from the double bond are said to have –R-effect.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




