
Atomicity of chlorine and argon is:
A)Diatomic and monatomic
B)monoatomic and diatomic
C)monoatomic and monatomic
D)diatomic and diatomic
Answer
585.9k+ views
Hint: Atomicity is defined as the total number of atoms that constitute a molecule. For example, each molecule of oxygen ($O_{2}$) is composed of two oxygen atoms. So the atomicity of oxygen is 2. In older contexts, the term atomicity is sometimes used in the same sense as valency.
Complete step-by-step answer:
Atomicity is defined as the total number of atoms that constitute a molecule.
On the basis of atomicity, molecules can be classified as :
-Monoatomic-composed of 1 atom
-Diatomic-composed of 2 atoms
-Triatomic-composed of 3 atoms
-Polyatomic-composed of 3 or more atoms
-Discussing option A, it is said that chlorine molecule is diatomic and argon is monoatomic. But, we know that the chlorine gas as a molecule is $Cl_{2}$. Argon being a noble gas is Ar in its ground state, thus mono atomic.
-Discussing option B, it is said that chlorine molecule is monatomic and argon is diatomic. But, we know that the chlorine gas as a molecule is $Cl_{2}$. Argon being a noble gas is Ar in its ground state, thus mono atomic.
-Discussing option C, it is said that chlorine molecule is monatomic and argon is monoatomic. But, we know that the chlorine gas as a molecule is $Cl_{2}$. Argon being a noble gas is Ar in its ground state, thus mono atomic.
-Discussing option D, it is said that chlorine molecule is diatomic and argon is diatomic. But, we know that the chlorine gas as a molecule is $Cl_{2}$. Argon being a noble gas is Ar in its ground state, thus mono atomic.
-Since there is an octet configuration in Argon, thus it is a stable molecule as monatomic.
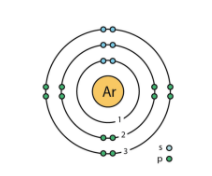
- Chlorine has 17 electrons intotal. Thus it needs one more electron in its outer orbital in order to achieve octet configuration. Hence it reacts with each other. 2 atoms of chlorine bonds and forms one chlorine molecule.
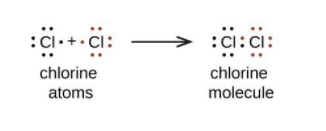
Clearly, the answer is A.
Note: Thus, it is understood that the atomicity of any molecule is based on the factor about the stable configuration of any molecule. Any molecule always tries to achieve its nearest and most stable electronic configuration.
Complete step-by-step answer:
Atomicity is defined as the total number of atoms that constitute a molecule.
On the basis of atomicity, molecules can be classified as :
-Monoatomic-composed of 1 atom
-Diatomic-composed of 2 atoms
-Triatomic-composed of 3 atoms
-Polyatomic-composed of 3 or more atoms
-Discussing option A, it is said that chlorine molecule is diatomic and argon is monoatomic. But, we know that the chlorine gas as a molecule is $Cl_{2}$. Argon being a noble gas is Ar in its ground state, thus mono atomic.
-Discussing option B, it is said that chlorine molecule is monatomic and argon is diatomic. But, we know that the chlorine gas as a molecule is $Cl_{2}$. Argon being a noble gas is Ar in its ground state, thus mono atomic.
-Discussing option C, it is said that chlorine molecule is monatomic and argon is monoatomic. But, we know that the chlorine gas as a molecule is $Cl_{2}$. Argon being a noble gas is Ar in its ground state, thus mono atomic.
-Discussing option D, it is said that chlorine molecule is diatomic and argon is diatomic. But, we know that the chlorine gas as a molecule is $Cl_{2}$. Argon being a noble gas is Ar in its ground state, thus mono atomic.
-Since there is an octet configuration in Argon, thus it is a stable molecule as monatomic.

- Chlorine has 17 electrons intotal. Thus it needs one more electron in its outer orbital in order to achieve octet configuration. Hence it reacts with each other. 2 atoms of chlorine bonds and forms one chlorine molecule.

Clearly, the answer is A.
Note: Thus, it is understood that the atomicity of any molecule is based on the factor about the stable configuration of any molecule. Any molecule always tries to achieve its nearest and most stable electronic configuration.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




