
Atomicity to white or yellow phosphorus is 4 and it is represented as ${ P }_{ 4 }$ molecule. Calculate the value of the expression $\dfrac { x.y }{ z }$ regarding this molecule.
Where,
x = total number of vertex angles in ${ P }_{ 4 }$ molecule
y = total number of lone pairs in ${ P }_{ 4 }$ molecule
z = total number of P-P bonds in ${ P }_{ 4 }$ molecule
Answer
600.6k+ views
Hint: In this question, it is necessary that you first draw the structure of a ${ P }_{ 4 }$ molecule. Then try to find the value of each quantity given in the question and finally apply those in the formula given. You will easily get the answer to your question.
Complete step by step answer:
First, we should look at the structure of a phosphorus molecule.
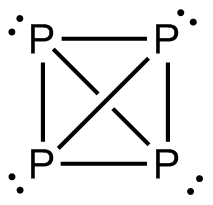
Atomicity of white or yellow phosphorus is 4 and it is represented as ${ P }_{ 4 }$ molecule.
Where x: Total number of vertex angles in ${ P }_{ 4 }$ molecule = number of P atoms $\times$ number of vertex angles per P atom = 4 $\times$ 3 = 12
y: Total number of lone pairs in ${ P }_{ 4 }$ molecule = number of P atoms $\times$ number of lone pairs per P atom = 4 $\times$ 1 = 4
z: Total number of P-P bonds in ${ P }_{ 4 }$ molecule = 6
Now put the value in the given expression = $\dfrac { x.y }{ z }$
This will be equal to $\dfrac { 12\times 4 }{ 6 }$ = 8
Therefore, we can say that the value of the expression $\dfrac { x.y }{ z }$ regarding this molecule is 8.
Note: You should also know that White phosphorus is a translucent waxy solid that quickly becomes yellow when exposed to light. For this reason, it is also called yellow phosphorus. It glows greenish in the dark (when exposed to oxygen) and is highly flammable and pyrophoric (self-igniting) upon contact with air.
Complete step by step answer:
First, we should look at the structure of a phosphorus molecule.

Atomicity of white or yellow phosphorus is 4 and it is represented as ${ P }_{ 4 }$ molecule.
Where x: Total number of vertex angles in ${ P }_{ 4 }$ molecule = number of P atoms $\times$ number of vertex angles per P atom = 4 $\times$ 3 = 12
y: Total number of lone pairs in ${ P }_{ 4 }$ molecule = number of P atoms $\times$ number of lone pairs per P atom = 4 $\times$ 1 = 4
z: Total number of P-P bonds in ${ P }_{ 4 }$ molecule = 6
Now put the value in the given expression = $\dfrac { x.y }{ z }$
This will be equal to $\dfrac { 12\times 4 }{ 6 }$ = 8
Therefore, we can say that the value of the expression $\dfrac { x.y }{ z }$ regarding this molecule is 8.
Note: You should also know that White phosphorus is a translucent waxy solid that quickly becomes yellow when exposed to light. For this reason, it is also called yellow phosphorus. It glows greenish in the dark (when exposed to oxygen) and is highly flammable and pyrophoric (self-igniting) upon contact with air.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




