
Available chlorine in a good sample of bleaching powder is:
A. 75%
B. 20-25%
C. 50-75%
D. 35-38%
Answer
578.1k+ views
Hint: In a good sample of bleaching powder chlorine is present in the form of chloride and hypochlorite. The amount of chlorine is variable in quantity but is not present in excess in the bleaching powder.
Complete step by step solution:
In order to answer our question, let's get to know some things about bleaching powder. Bleaching powder is an inorganic compound and its chemical name is calcium hypochlorite, and it has the molecular formula $Ca{{(ClO)}_{2}}$, also along with some amount of calcium chloride. It is used both for bleaching purposes, as well as in the treatment of water. It may look yellow, but in the laboratory, where it is prepared, it is seen as a white solid. It decomposes in moist air and generally, has a strong smell of chlorine. This is how the structure of bleaching powder looks like:
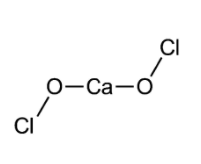
Following is the reaction which shows the industrial preparation of bleaching powder:
$2C{{l}_{2}}+2Ca{{(OH)}_{2}}->Ca{{(ClO)}_{2}}+CaC{{l}_{2}}+2{{H}_{2}}O$
$Ca{{(OH)}_{2}}$ is also called slaked lime, and is an important ingredient in the production of bleaching powder.
Now, in every reaction, the weights of the chlorine and other compounds are given. Mathematically, the mass of chlorine comes out to be:
$\%$ $of$ $Cl=$$\dfrac{{M_{Cl}}\times 100}{W(gm)}$
Since the values of mass and weight are different for different industries, we cannot find the exact value of the percentage of chlorine. However, it is noticed that chlorine is present in a quantity of about 20-25% in bleaching powder.
So, we obtain our answer to be option B.
Note: Bleaching powder is not soluble in all types of water. It is only soluble in soft to medium water. Hard water contains salts which hinder the solubility of bleaching powder. Bleaching powder can be present in both dry and hydrous forms.
Complete step by step solution:
In order to answer our question, let's get to know some things about bleaching powder. Bleaching powder is an inorganic compound and its chemical name is calcium hypochlorite, and it has the molecular formula $Ca{{(ClO)}_{2}}$, also along with some amount of calcium chloride. It is used both for bleaching purposes, as well as in the treatment of water. It may look yellow, but in the laboratory, where it is prepared, it is seen as a white solid. It decomposes in moist air and generally, has a strong smell of chlorine. This is how the structure of bleaching powder looks like:

Following is the reaction which shows the industrial preparation of bleaching powder:
$2C{{l}_{2}}+2Ca{{(OH)}_{2}}->Ca{{(ClO)}_{2}}+CaC{{l}_{2}}+2{{H}_{2}}O$
$Ca{{(OH)}_{2}}$ is also called slaked lime, and is an important ingredient in the production of bleaching powder.
Now, in every reaction, the weights of the chlorine and other compounds are given. Mathematically, the mass of chlorine comes out to be:
$\%$ $of$ $Cl=$$\dfrac{{M_{Cl}}\times 100}{W(gm)}$
Since the values of mass and weight are different for different industries, we cannot find the exact value of the percentage of chlorine. However, it is noticed that chlorine is present in a quantity of about 20-25% in bleaching powder.
So, we obtain our answer to be option B.
Note: Bleaching powder is not soluble in all types of water. It is only soluble in soft to medium water. Hard water contains salts which hinder the solubility of bleaching powder. Bleaching powder can be present in both dry and hydrous forms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




