
Azoxybenzene can be obtained by the treatment of nitro-benzene with:
A.\[{{O}_{2}}\]
B.\[{{H}_{2}}/Pt\]
C.\[N{{a}_{3}}A{{s}_{2}}{{O}_{3}}/NaOH\]
D.\[Zn/NaOH\]
Answer
567.6k+ views
Hint:Azobenzene is made by using two molecules of nitrobenzene, and the two nitrogens are attached to each other by a double bond.
In this conversion process, the reduction of nitrobenzene takes place.
Complete step by step answer:
The structure of nitrobenzene has a nitro group attached to the benzene ring. Nitrobenzene is an organic compound which has the chemical formula \[{{C}_{6}}{{H}_{5}}N{{O}_{2}}\]. It is water-insoluble, and has pale yellow oil type texture with an almond-like odour. It freezes in order to give greenish-yellow crystals. It is produced on a huge scale from benzene as a precursor to aniline. In the laboratory, it is occasionally used as a solvent, especially for electrophilic reagents.
The word azoxy means that the compound contains a nitrogen-nitrogen double bond as well as a nitrogen oxygen bond. They are considered as \[N-oxides\] of azo compounds. Azoxy compounds are \[1,3-dipoles\]. They undergo \[1,3\text{ }dipolar\] cycloaddition with double bonds. Most compounds containing azoxy have aryl substituents. They are normally prepared by reduction of nitro compounds, such as the reduction of nitrobenzene with the help of arsenous oxide to azoxybenzene.
Azoxybenzene is an organic compound with the formula \[{{C}_{6}}{{H}_{5}}N\left( O \right)N{{C}_{6}}{{H}_{5}}\]. It is a yellow, low-melting solid. The molecule has a planar \[{{C}_{2}}{{N}_{2}}O\] core.
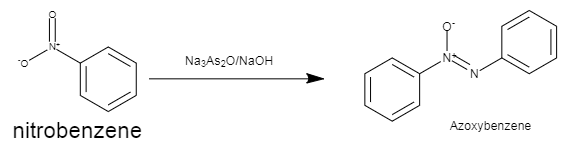
The structure of nitrobenzene and azobenzene is shown the given diagram below.
This diagram depicts the conversion of nitrobenzene to azoxybenzene by the use of \[N{{a}_{3}}A{{s}_{2}}{{O}_{3}}/NaOH\] as a reagent.
A detailed mechanism of the reaction is explained below.
In this process, Nitrobenzene first reduces to nitrobenzene and \[N-hydroxyaniline\]. Some of the nitrobenzene could further get reduced to aniline and \[N-phenylhydroxylamine\], both of which are termed as nucleophiles and can react with the nitroso \[N=O\] bond (which is analogous to a carbonyl). Elimination of water in both reactions results in formation of azobenzene and azobenzene respectively.
So the correct option is C.
Note:Nitrobenzene is a toxic chemical with a number of harmful effects
-Acute inhalation, or oral, and dermal exposure to nitrobenzene in human beings produces a condition called methemoglobinemia, in which hemoglobin in blood, is converted to methemoglobin, which results in lowering in the amount of oxygen released to the tissues of the human body.
In this conversion process, the reduction of nitrobenzene takes place.
Complete step by step answer:
The structure of nitrobenzene has a nitro group attached to the benzene ring. Nitrobenzene is an organic compound which has the chemical formula \[{{C}_{6}}{{H}_{5}}N{{O}_{2}}\]. It is water-insoluble, and has pale yellow oil type texture with an almond-like odour. It freezes in order to give greenish-yellow crystals. It is produced on a huge scale from benzene as a precursor to aniline. In the laboratory, it is occasionally used as a solvent, especially for electrophilic reagents.
The word azoxy means that the compound contains a nitrogen-nitrogen double bond as well as a nitrogen oxygen bond. They are considered as \[N-oxides\] of azo compounds. Azoxy compounds are \[1,3-dipoles\]. They undergo \[1,3\text{ }dipolar\] cycloaddition with double bonds. Most compounds containing azoxy have aryl substituents. They are normally prepared by reduction of nitro compounds, such as the reduction of nitrobenzene with the help of arsenous oxide to azoxybenzene.
Azoxybenzene is an organic compound with the formula \[{{C}_{6}}{{H}_{5}}N\left( O \right)N{{C}_{6}}{{H}_{5}}\]. It is a yellow, low-melting solid. The molecule has a planar \[{{C}_{2}}{{N}_{2}}O\] core.
The structure of nitrobenzene and azobenzene is shown the given diagram below.
This diagram depicts the conversion of nitrobenzene to azoxybenzene by the use of \[N{{a}_{3}}A{{s}_{2}}{{O}_{3}}/NaOH\] as a reagent.

A detailed mechanism of the reaction is explained below.
In this process, Nitrobenzene first reduces to nitrobenzene and \[N-hydroxyaniline\]. Some of the nitrobenzene could further get reduced to aniline and \[N-phenylhydroxylamine\], both of which are termed as nucleophiles and can react with the nitroso \[N=O\] bond (which is analogous to a carbonyl). Elimination of water in both reactions results in formation of azobenzene and azobenzene respectively.
So the correct option is C.
Note:Nitrobenzene is a toxic chemical with a number of harmful effects
-Acute inhalation, or oral, and dermal exposure to nitrobenzene in human beings produces a condition called methemoglobinemia, in which hemoglobin in blood, is converted to methemoglobin, which results in lowering in the amount of oxygen released to the tissues of the human body.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




