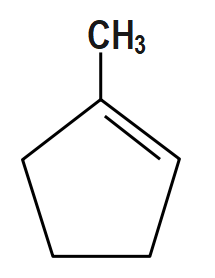
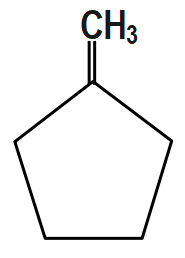
Answer
406.5k+ views
Hint :We know that in order to know how to solve a stoichiometry problem, we must first know what a stoichiometry is. Stoichiometry is the relationship between the quantities of the reactants and the products. If we know the quantity of the reactant it will be easy to determine the product.
Complete Step By Step Answer:
Let us understand deeply about stoichiometry. Stoichiometry will be based on the Law of conservation of mass. That means that the total mass of the reactants will be equal to the total mass of the products. If we know the amount of the reactants, from that we can calculate the amount of the product. We can determine the quantity of the product empirically from the quantity of the reactant.
We have to remember that there are four steps for solving a stoichiometric problem.
First thing is that we have to write a balanced equation. We have to always keep in mind that the constituent part of a chemical reaction is neither destroyed nor lost. The reactions yield must correspond to the reactant.
The next step is to convert the unit into the moles. When a unit is converted into moles it will involve the conversion factor. The main aim of the conversion factor is to convert units such as the grams, volume of a gas into moles or vice versa.
Now by using the mole ratio, we have to calculate the other substance (product).
Here, First step is an example of the Wittig reaction and then in addition to the base $ Bu-Li, $ it gives a thermodynamic product.
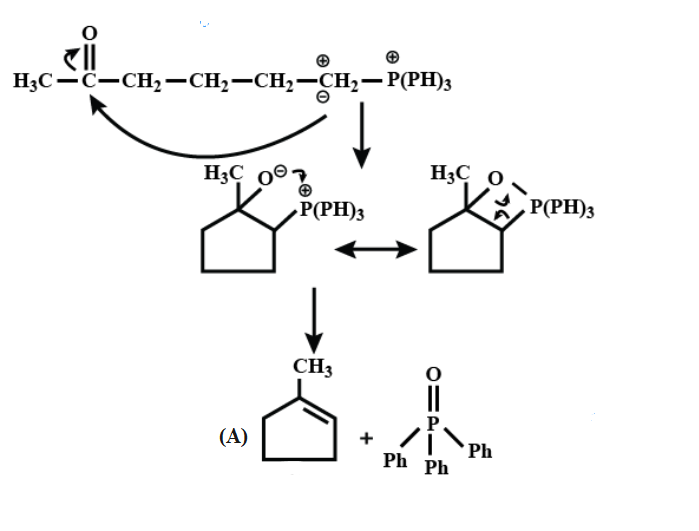
On further continuing the reaction;
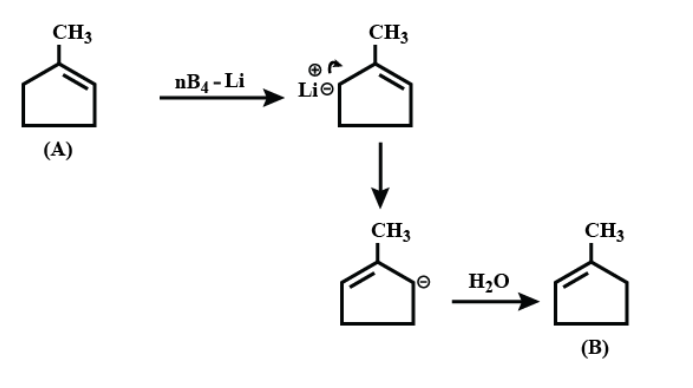
Therefore the correct answer is option A.
Note :
Remember that we have to remember that stoichiometry compounds and non-stoichiometry compounds are different from one another. Non-stoichiometry compound is mostly an inorganic compound whose proportions of the elemental composition cannot be determined by the ratio of the natural number.
Complete Step By Step Answer:
Let us understand deeply about stoichiometry. Stoichiometry will be based on the Law of conservation of mass. That means that the total mass of the reactants will be equal to the total mass of the products. If we know the amount of the reactants, from that we can calculate the amount of the product. We can determine the quantity of the product empirically from the quantity of the reactant.
We have to remember that there are four steps for solving a stoichiometric problem.
First thing is that we have to write a balanced equation. We have to always keep in mind that the constituent part of a chemical reaction is neither destroyed nor lost. The reactions yield must correspond to the reactant.
The next step is to convert the unit into the moles. When a unit is converted into moles it will involve the conversion factor. The main aim of the conversion factor is to convert units such as the grams, volume of a gas into moles or vice versa.
Now by using the mole ratio, we have to calculate the other substance (product).
Here, First step is an example of the Wittig reaction and then in addition to the base $ Bu-Li, $ it gives a thermodynamic product.

On further continuing the reaction;

Therefore the correct answer is option A.
Note :
Remember that we have to remember that stoichiometry compounds and non-stoichiometry compounds are different from one another. Non-stoichiometry compound is mostly an inorganic compound whose proportions of the elemental composition cannot be determined by the ratio of the natural number.
Recently Updated Pages
ABC is a right angled triangular plate of uniform thickness class 11 phy sec 1 JEE_Main

The linear velocity perpendicular to the radius vector class 11 physics JEE_Main

The normality of 03 M phosphorus acid H3PO3 is class 11 chemistry NEET_UG

The total work done on a particle is equal to the change class 11 physics JEE_Main

A cylindrical tube open at both ends has a fundamental class 11 physics JEE_Main

For which of the following reactions H is equal to class 11 chemistry JEE_Main

Trending doubts
Which is the longest day and shortest night in the class 11 sst CBSE

Who was the Governor general of India at the time of class 11 social science CBSE

Why is steel more elastic than rubber class 11 physics CBSE

Difference between Prokaryotic cell and Eukaryotic class 11 biology CBSE

Define the term system surroundings open system closed class 11 chemistry CBSE

State and prove Bernoullis theorem class 11 physics CBSE