
Balance the following redox reaction by ion electron method:
\[C{{r}_{2}}{{O}_{7}}^{2-}+S{{O}_{2}}(g)\to C{{r}^{3+}}(aq)+S{{O}_{4}}^{2-}(aq)\]
Answer
593.1k+ views
Hint: Split the oxidation half-reaction and reduction half-reaction. In the acidic medium, H atoms are balanced by adding hydrogen ions to the side deficient in H atoms. The total charge on either side of the equation must be equal.
Complete answer:
Ion electron method: This method of balancing the reaction is based on the principle that electrons lost during oxidation half-reaction of any redox reaction are equal to the electrons gained during the reduction half-reaction.
Step by step we will solve the reaction:
Step 1: Write the skeleton equation for the given reaction.
\[C{{r}_{2}}{{O}_{7}}^{2-}+S{{O}_{2}}(g)\to C{{r}^{3+}}(aq)+S{{O}_{4}}^{2-}(aq)\text{ (i)}\]
Step 2: Find out the elements which change the oxidation number (O.N)
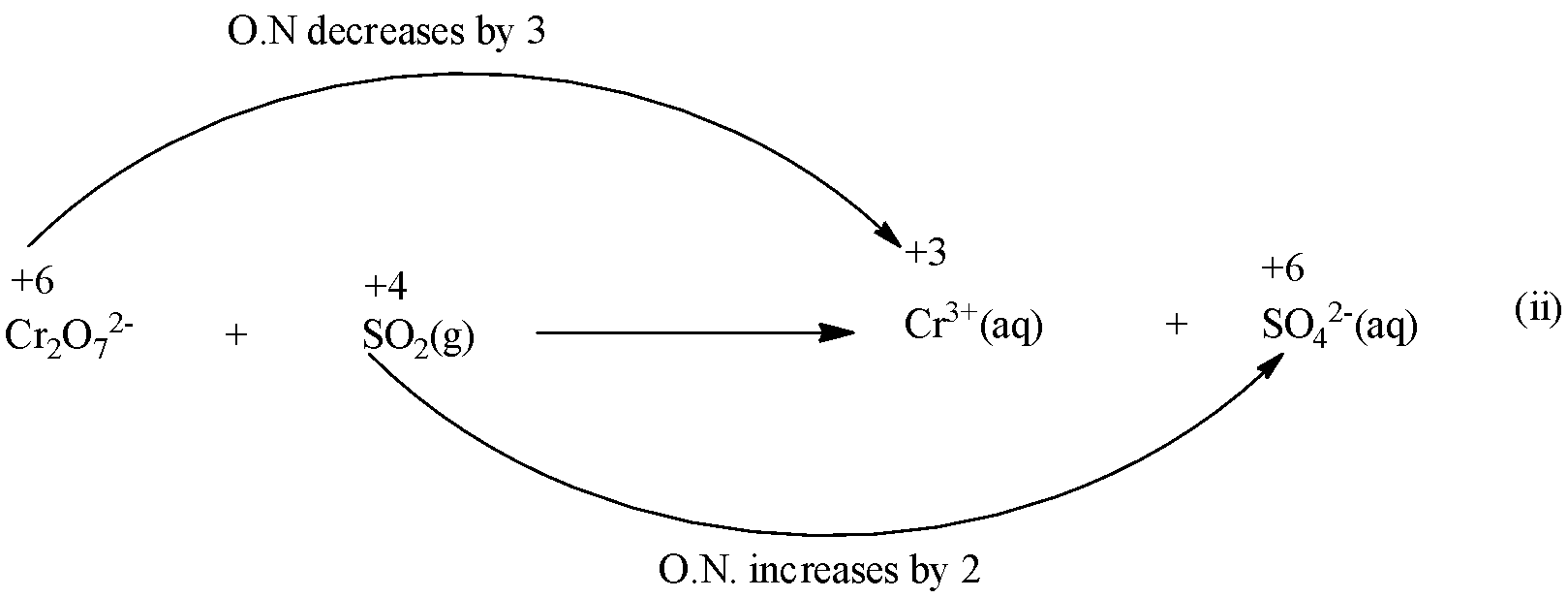
Here, the oxidation number of Cr decreases from +6 in\[C{{r}_{2}}{{O}_{7}}^{2-}\] to +3 in\[C{{r}^{3+}}\] while that of S increases from +4 in\[S{{O}_{2}}\] to +6 in\[S{{O}_{4}}^{2-}\] .
Step 3: find out the total increases and decrease in O.N.
Since there are two Cr atoms in L.H.S and only one on R.H.S., therefore, multiply \[C{{r}^{3+}}\] on R.H.S. of equation (i) by 2 and thus the total decrease in O.N of Cr is 2*3= 6.
Further, since there is only one S atom on either side of equation (ii), therefore, the total increase in O.N of S is 2.
Step 4: balance the increase or decrease in O.N.
Since the total increase in O.N. is 2 and decrease is 6, therefore, multiply \[S{{O}_{2}}\] on L.H.S and \[S{{O}_{4}}^{2-}\] on R.H.S of the equation by 3. The combining equation is:
\[C{{r}_{2}}{{O}_{7}}^{2-}+3S{{O}_{2}}(g)\to 2C{{r}^{3+}}(aq)+3S{{O}_{4}}^{2-}(aq)\]
Step 5: balance all other atoms than H and O
No need to balance because Cr and S are already balanced.
Step 6: balance O by adding molecules.
In the L.H.S there are 13 oxygen atoms and on the R.H.S there are 12 oxygen atoms. So, add one\[{{H}_{2}}O\] molecule to R.H.S.
\[C{{r}_{2}}{{O}_{7}}^{2-}+3S{{O}_{2}}(g)\to 2C{{r}^{3+}}(aq)+3S{{O}_{4}}^{2-}(aq)+{{H}_{2}}O(aq)\]
Step 7: balance the H atoms by adding ions since the reaction occurs in the acidic medium.
Since there are 2 hydrogen atoms on the R.H.S, we have to add 2 ions on the L.H.S.
\[C{{r}_{2}}{{O}_{7}}^{2-}+3S{{O}_{2}}(g)+2{{H}^{+}}(aq)\to 2C{{r}^{3+}}(aq)+3S{{O}_{4}}^{2-}(aq)+{{H}_{2}}O(aq)\]
Thus, the equation is now balanced.
Note: You should always check the charge on both sides. For balance you can only use water molecules and don't use oxygen atoms to balance the equation. If the equation is in basic medium hydroxyl ion is used to balance the hydrogen atoms.
Complete answer:
Ion electron method: This method of balancing the reaction is based on the principle that electrons lost during oxidation half-reaction of any redox reaction are equal to the electrons gained during the reduction half-reaction.
Step by step we will solve the reaction:
Step 1: Write the skeleton equation for the given reaction.
\[C{{r}_{2}}{{O}_{7}}^{2-}+S{{O}_{2}}(g)\to C{{r}^{3+}}(aq)+S{{O}_{4}}^{2-}(aq)\text{ (i)}\]
Step 2: Find out the elements which change the oxidation number (O.N)

Here, the oxidation number of Cr decreases from +6 in\[C{{r}_{2}}{{O}_{7}}^{2-}\] to +3 in\[C{{r}^{3+}}\] while that of S increases from +4 in\[S{{O}_{2}}\] to +6 in\[S{{O}_{4}}^{2-}\] .
Step 3: find out the total increases and decrease in O.N.
Since there are two Cr atoms in L.H.S and only one on R.H.S., therefore, multiply \[C{{r}^{3+}}\] on R.H.S. of equation (i) by 2 and thus the total decrease in O.N of Cr is 2*3= 6.
Further, since there is only one S atom on either side of equation (ii), therefore, the total increase in O.N of S is 2.
Step 4: balance the increase or decrease in O.N.
Since the total increase in O.N. is 2 and decrease is 6, therefore, multiply \[S{{O}_{2}}\] on L.H.S and \[S{{O}_{4}}^{2-}\] on R.H.S of the equation by 3. The combining equation is:
\[C{{r}_{2}}{{O}_{7}}^{2-}+3S{{O}_{2}}(g)\to 2C{{r}^{3+}}(aq)+3S{{O}_{4}}^{2-}(aq)\]
Step 5: balance all other atoms than H and O
No need to balance because Cr and S are already balanced.
Step 6: balance O by adding molecules.
In the L.H.S there are 13 oxygen atoms and on the R.H.S there are 12 oxygen atoms. So, add one\[{{H}_{2}}O\] molecule to R.H.S.
\[C{{r}_{2}}{{O}_{7}}^{2-}+3S{{O}_{2}}(g)\to 2C{{r}^{3+}}(aq)+3S{{O}_{4}}^{2-}(aq)+{{H}_{2}}O(aq)\]
Step 7: balance the H atoms by adding ions since the reaction occurs in the acidic medium.
Since there are 2 hydrogen atoms on the R.H.S, we have to add 2 ions on the L.H.S.
\[C{{r}_{2}}{{O}_{7}}^{2-}+3S{{O}_{2}}(g)+2{{H}^{+}}(aq)\to 2C{{r}^{3+}}(aq)+3S{{O}_{4}}^{2-}(aq)+{{H}_{2}}O(aq)\]
Thus, the equation is now balanced.
Note: You should always check the charge on both sides. For balance you can only use water molecules and don't use oxygen atoms to balance the equation. If the equation is in basic medium hydroxyl ion is used to balance the hydrogen atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




