
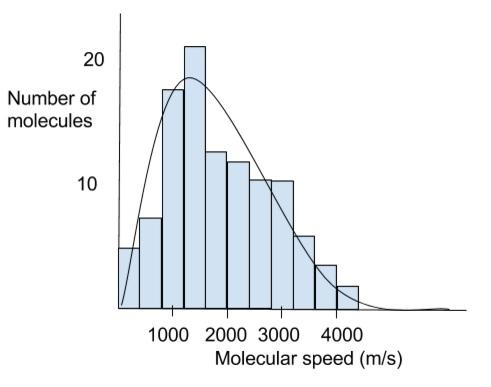
Based on the Maxwell Boltzmann graph showing the number of molecules for a gas versus the speed of the molecules, which of the following indicates that a unimolecular reaction will occur with a faster rate?
A. Dealing with a gas indicates that the molecule will only react when the molecules collide with the container due to wide distribution of the particles.
B. Increasing the pressure of the system will force the molecules closer together increasing the number of collisions resulting in a faster reaction.
C. A homogeneous catalyst must be used to start any unimolecular reaction involving gases.
D. Decreasing the temperature of the system will increase the average kinetic energy of the molecules resulting in a greater number of collisions needed to start the reaction

Answer
566.7k+ views
Hint: If a fraction of molecules having particular speed are plotted against their corresponding speeds at any particular temperature then a curve is obtained that distribution of speed is known as Maxwell Boltzmann distribution and graph is represented by Maxwell Boltzmann graph.
Complete Solution :
- At any definite temperature different molecules of gases have different speeds but due to continuous collision their speeds will change; it may be increasing or decreasing but their particular speed remains constant at any constant pressure and the curve plotted against their corresponding speeds at any particular temperature is known by Maxwell Boltzmann distribution curve.
- In general we can say that the Maxwell distribution curve tells us about the speed of the molecules at a particular temperature. The speed of the largest number of molecules is the most probable speed. And if we talk about the effect of temperature on the speed that the temperature will increase with the increase in most probable speed. Also it can be considered that the curve flattens when the temperature increases. Thus we can easily conclude that at high temperatures the larger number of molecules is moving at greater speed.
So, the correct answer is “Option A”.
Note: Like temperature there is also an effect of nature of gas depending on the curve which can be described as lighter gases will have higher probable speed as compared to heavier one i.e. we can say that lighter gases can move easily as compared to heavier gases.
Complete Solution :
- At any definite temperature different molecules of gases have different speeds but due to continuous collision their speeds will change; it may be increasing or decreasing but their particular speed remains constant at any constant pressure and the curve plotted against their corresponding speeds at any particular temperature is known by Maxwell Boltzmann distribution curve.
- In general we can say that the Maxwell distribution curve tells us about the speed of the molecules at a particular temperature. The speed of the largest number of molecules is the most probable speed. And if we talk about the effect of temperature on the speed that the temperature will increase with the increase in most probable speed. Also it can be considered that the curve flattens when the temperature increases. Thus we can easily conclude that at high temperatures the larger number of molecules is moving at greater speed.
So, the correct answer is “Option A”.
Note: Like temperature there is also an effect of nature of gas depending on the curve which can be described as lighter gases will have higher probable speed as compared to heavier one i.e. we can say that lighter gases can move easily as compared to heavier gases.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




