
Benzalacetone is the product of mixed aldol condensation between benzaldehyde $({C_6}{H_5}CH = O)$ and acetone $[{(C{H_3})_2}C\,\, + \,\,O]$ . What is its structure?
A) ${C_6}{H_5}CH = CHC(O)(C{H_3})$
B) ${C_6}{H_5}CH = C{(C{H_3})_2}$
C) ${C_6}{H_5}C(O)CH = CHC{H_3}$
D) ${C_6}{H_5}C{H_2}C(O)CH = C{H_2}$
Answer
560.7k+ views
Hint: To solve this question, we must first understand the whole concept of Aldol Condensation. Then we need to assess the information of the reaction and its occurrence mechanism and then by carrying out the reaction by following the mechanism correctly and then only we can conclude the correct answer.
Complete answer:
Before we move forward with the solution of this given question, let us first understand some basic concepts:
Aldol condensation: is a condensation reaction in organic chemistry in which an enol or an enolate ion reacts with a carbonyl compound to form a $\beta - $ hydroxy aldehyde or $\beta - $ hydroxy ketone (an aldol reaction), followed by dehydration to give a conjugated enone.
Aldol condensation occurs in aldehydes having $\alpha - $ hydrogen with a dilute base to give $\beta - $ hydroxy aldehydes called aldols. This reaction is most commonly known as aldol condensation. If the condensation reaction occurs between two different carbonyl compounds it is called crossed aldol condensation.
Step 1: We will carry out the given reaction according to the mechanism of Aldol condensation:
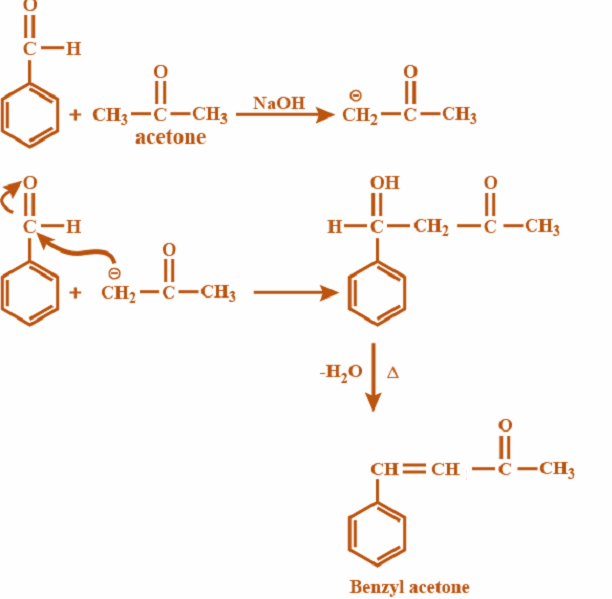
A mixture of products are possible in cross aldol reaction but major is as above. This is $\alpha ,\beta $ unsaturated ketone.
So from the reaction presented above, the required product is ${C_6}{H_5}CH = CHC(O)(C{H_3})$
Therefore the correct option is option A..
Note:The Aldox process is an industrial variation of the aldol condensation reaction for the direct conversion of syngas and propene into $2 - $ ethyl hexanol. This product is formed via the hydroformylation of the reactants into butyraldehyde, its subsequent aldol condensation into $2 - $ ethyl hexenal, and the hydrogenation of this intermediate into $2 - $ ethyl hexanol.
Complete answer:
Before we move forward with the solution of this given question, let us first understand some basic concepts:
Aldol condensation: is a condensation reaction in organic chemistry in which an enol or an enolate ion reacts with a carbonyl compound to form a $\beta - $ hydroxy aldehyde or $\beta - $ hydroxy ketone (an aldol reaction), followed by dehydration to give a conjugated enone.
Aldol condensation occurs in aldehydes having $\alpha - $ hydrogen with a dilute base to give $\beta - $ hydroxy aldehydes called aldols. This reaction is most commonly known as aldol condensation. If the condensation reaction occurs between two different carbonyl compounds it is called crossed aldol condensation.
Step 1: We will carry out the given reaction according to the mechanism of Aldol condensation:

A mixture of products are possible in cross aldol reaction but major is as above. This is $\alpha ,\beta $ unsaturated ketone.
So from the reaction presented above, the required product is ${C_6}{H_5}CH = CHC(O)(C{H_3})$
Therefore the correct option is option A..
Note:The Aldox process is an industrial variation of the aldol condensation reaction for the direct conversion of syngas and propene into $2 - $ ethyl hexanol. This product is formed via the hydroformylation of the reactants into butyraldehyde, its subsequent aldol condensation into $2 - $ ethyl hexenal, and the hydrogenation of this intermediate into $2 - $ ethyl hexanol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




