
Benzaldehyde on reaction with acetophenone in presence of sodium hydroxide solution gives:
A. ${{C}_{6}}{{H}_{5}}CH=CHCO{{C}_{6}}{{H}_{5}}$
B. ${{C}_{6}}{{H}_{5}}COC{{H}_{2}}{{C}_{6}}{{H}_{5}}$
C. ${{C}_{6}}{{H}_{5}}CH=CH{{C}_{6}}{{H}_{5}}$
D. ${{C}_{6}}{{H}_{5}}CH(OH){{C}_{6}}{{H}_{5}}$
Answer
515.1k+ views
Hint: Benzaldehyde is an organic compound consisting of a benzene ring with a formyl substituent. The molecular formula\[{{C}_{6}}{{H}_{5}}CHO\]. It is a colorless liquid having an almond like odor. It can be extracted from a number of other natural sources. Synthetic benzaldehyde is the flavoring agent which is used to flavor cakes and other baked goods.
Complete answer:
The reaction in which benzaldehyde reacts with acetophenone in presence of sodium hydroxide solution is known as cross aldol condensation reaction in which benzaldehyde which is aromatic aldehyde compound reacts with acetophenone which aliphatic alkyl ketone and sodium hydroxide acts as an catalyst here.
The reaction given above generally based on the concept of aldol condensation which occurs in aldehydes and the necessary condition for aldol condensation is presence of $\alpha $ hydrogens with a dilute base which gives $\beta $ hydroxy aldehydes known by the name aldols. Aldol condensations play a very important role in organic synthesis as it creates a path to form carbon-carbon bonds. When the same type of reaction occurs with two different types of carbonyl compounds then this type of reactions are known by the name cross aldol condensation. Reaction can be shown as follows:
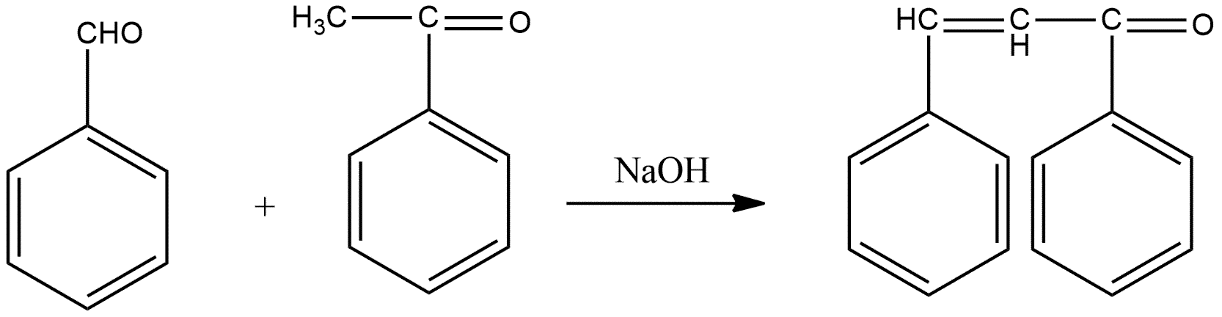
Hence option A is the correct answer.
Note:
Benzaldehyde can be oxidized to benzoic acid. The boiling point of benzoic acid is much higher than that of benzaldehyde; it can be purified by a simple process called distillation. Benzyl alcohol can be formed from benzaldehyde by means of hydrogenation.
Complete answer:
The reaction in which benzaldehyde reacts with acetophenone in presence of sodium hydroxide solution is known as cross aldol condensation reaction in which benzaldehyde which is aromatic aldehyde compound reacts with acetophenone which aliphatic alkyl ketone and sodium hydroxide acts as an catalyst here.
The reaction given above generally based on the concept of aldol condensation which occurs in aldehydes and the necessary condition for aldol condensation is presence of $\alpha $ hydrogens with a dilute base which gives $\beta $ hydroxy aldehydes known by the name aldols. Aldol condensations play a very important role in organic synthesis as it creates a path to form carbon-carbon bonds. When the same type of reaction occurs with two different types of carbonyl compounds then this type of reactions are known by the name cross aldol condensation. Reaction can be shown as follows:

Hence option A is the correct answer.
Note:
Benzaldehyde can be oxidized to benzoic acid. The boiling point of benzoic acid is much higher than that of benzaldehyde; it can be purified by a simple process called distillation. Benzyl alcohol can be formed from benzaldehyde by means of hydrogenation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




