
How is benzamide obtained from benzoic acid?
Answer
599.1k+ views
Hint: To draw the conversion of benzoic acid to benzamide we should know about the conditions that are required for production of benzamide. We should know that in benzamide it has an amine group attached to it.
Step by step answer:
Before drawing the conversion of benzoic acid to benzamide, we should know about benzamide. We should note that benzamide is a white solid with the chemical formula of \[{{C}_{6}}{{H}_{5}}C\left( O \right)N{{H}_{2}}\]. It is the simplest amide derivative of benzoic acid.
It has the following structure:
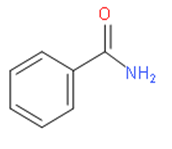
For the synthesis of benzamide from benzoic acid, we should use thionyl chloride (\[SOC{{l}_{2}}\])
with benzoic acid to convert it in benzyl chloride. In this reaction, we will replace OH to Cl then we
will use \[N{{H}_{3}}\] which upon reacting with benzyl chloride will give us benzamide. This
reaction will be as follows:

So, in the above reaction we had converted benzoic acid to benzoyl chloride. We should note that benzoyl chloride appears as a colourless fuming liquid with a pungent odour. We should note that benzoyl chloride is a typical acyl chloride. It reacts with alcohols to give the corresponding esters.
Similarly, it reacts with amines to give the amide. So, if we react with benzoyl chloride with amines it will give amide. We should know that we use thionyl chloride, because it is used as a chlorinating agent.
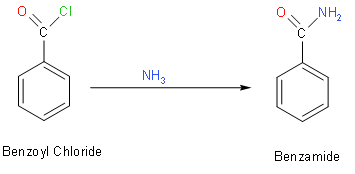
So, in the above reaction we had converted benzoic acid to benzamide.
Note: We can also prepare benzamide with the help of benzonitrile. When benzonitrile is mixed with sulphuric acid, a clear solution is rapidly obtained. We should then heat it for 20 minutes. When the hydrolysis reaction is complete the solution is cooled. The obtained precipitate is of crude benzamide.We should be extremely careful while making benzamide from thionyl chloride, because it is highly corrosive and toxic. Long-term inhalation of low concentrations or short-term inhalation of high concentrations has adverse health effects.
Step by step answer:
Before drawing the conversion of benzoic acid to benzamide, we should know about benzamide. We should note that benzamide is a white solid with the chemical formula of \[{{C}_{6}}{{H}_{5}}C\left( O \right)N{{H}_{2}}\]. It is the simplest amide derivative of benzoic acid.
It has the following structure:

For the synthesis of benzamide from benzoic acid, we should use thionyl chloride (\[SOC{{l}_{2}}\])
with benzoic acid to convert it in benzyl chloride. In this reaction, we will replace OH to Cl then we
will use \[N{{H}_{3}}\] which upon reacting with benzyl chloride will give us benzamide. This
reaction will be as follows:

So, in the above reaction we had converted benzoic acid to benzoyl chloride. We should note that benzoyl chloride appears as a colourless fuming liquid with a pungent odour. We should note that benzoyl chloride is a typical acyl chloride. It reacts with alcohols to give the corresponding esters.
Similarly, it reacts with amines to give the amide. So, if we react with benzoyl chloride with amines it will give amide. We should know that we use thionyl chloride, because it is used as a chlorinating agent.

So, in the above reaction we had converted benzoic acid to benzamide.
Note: We can also prepare benzamide with the help of benzonitrile. When benzonitrile is mixed with sulphuric acid, a clear solution is rapidly obtained. We should then heat it for 20 minutes. When the hydrolysis reaction is complete the solution is cooled. The obtained precipitate is of crude benzamide.We should be extremely careful while making benzamide from thionyl chloride, because it is highly corrosive and toxic. Long-term inhalation of low concentrations or short-term inhalation of high concentrations has adverse health effects.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




