
How can benzene be converted into acetophenone?
Answer
552k+ views
Hint:Benzene is an organic compound which is stabilized by resonance. It is a colourless and highly flammable liquid with a sweet smell. In Friedal crafts acylation, Lewis acid is used as a catalyst. Lewis acid is a substance that accepts non bonding electrons. In other words we can say that Lewis acid is an electron pair acceptor.
Complete step-by-step answer:In this question, Friedel craft acylation is used to get the desired product. In this reaction, addition of acyl group to aromatic compound takes place. Here, the catalyst Lewis acid and acid chloride is used to complete the reaction. the halogen that belongs to acyl halide forms a complex with Lewis acid and generates a high electrophilic acylium ion which is stabilized by resonance.
When benzene is reacted with acetyl chloride in presence of anhydrous aluminium chloride which is a Lewis acid, it results in the formation of acetophenone. In this reaction, when acetyl chloride is treated with anhydrous aluminium chloride, a positive charge is formed on the carbonyl carbon of acetyl chloride and tetrachloroaluminate anion is formed. After that the positive charge of the carbonyl carbon or the acyl group gets attached to the benzene which further reacts with tetrachloroaluminate and results in the formation of acetophenone.
Let us see the reaction:
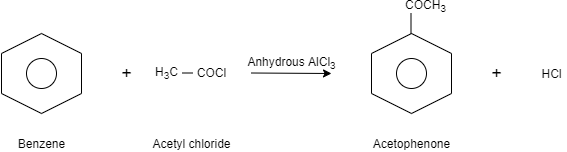
Note:There are some limitations of acylation reactions-
1.This reaction yields only ketoses. The reason behind this is formyl chloride decomposes into carbon monoxide and hydrogen chloride.
2.Aryl amines cannot be used in acylation reactions because they form highly unreactive complexe with Lewis acid.
Complete step-by-step answer:In this question, Friedel craft acylation is used to get the desired product. In this reaction, addition of acyl group to aromatic compound takes place. Here, the catalyst Lewis acid and acid chloride is used to complete the reaction. the halogen that belongs to acyl halide forms a complex with Lewis acid and generates a high electrophilic acylium ion which is stabilized by resonance.
When benzene is reacted with acetyl chloride in presence of anhydrous aluminium chloride which is a Lewis acid, it results in the formation of acetophenone. In this reaction, when acetyl chloride is treated with anhydrous aluminium chloride, a positive charge is formed on the carbonyl carbon of acetyl chloride and tetrachloroaluminate anion is formed. After that the positive charge of the carbonyl carbon or the acyl group gets attached to the benzene which further reacts with tetrachloroaluminate and results in the formation of acetophenone.
Let us see the reaction:

Note:There are some limitations of acylation reactions-
1.This reaction yields only ketoses. The reason behind this is formyl chloride decomposes into carbon monoxide and hydrogen chloride.
2.Aryl amines cannot be used in acylation reactions because they form highly unreactive complexe with Lewis acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




