
Benzene can be obtained by heating either benzoic acid with X or phenol with Y. X and Y are ________ respectively.
A. zinc dust and soda lime
B. soda lime and zinc dust
C. zinc dust and sodium hydroxide
D. soda lime and copper
Answer
590.4k+ views
Hint: Phenol to benzene is a radical reaction. While benzoic acid to benzene is a decarboxylation reaction. This occurs at low temperature and high temperature.
Complete step by step answer:
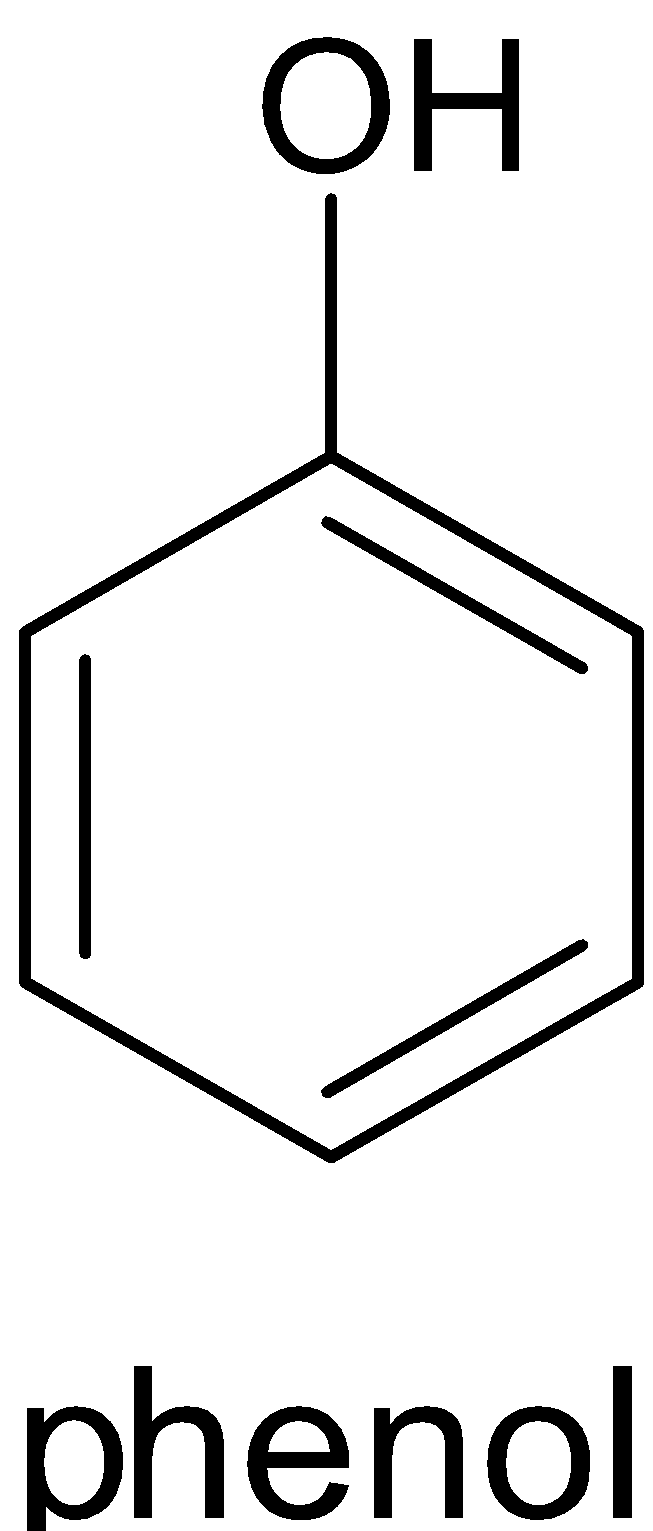 $\xrightarrow{{\text{Y}}}$
$\xrightarrow{{\text{Y}}}$
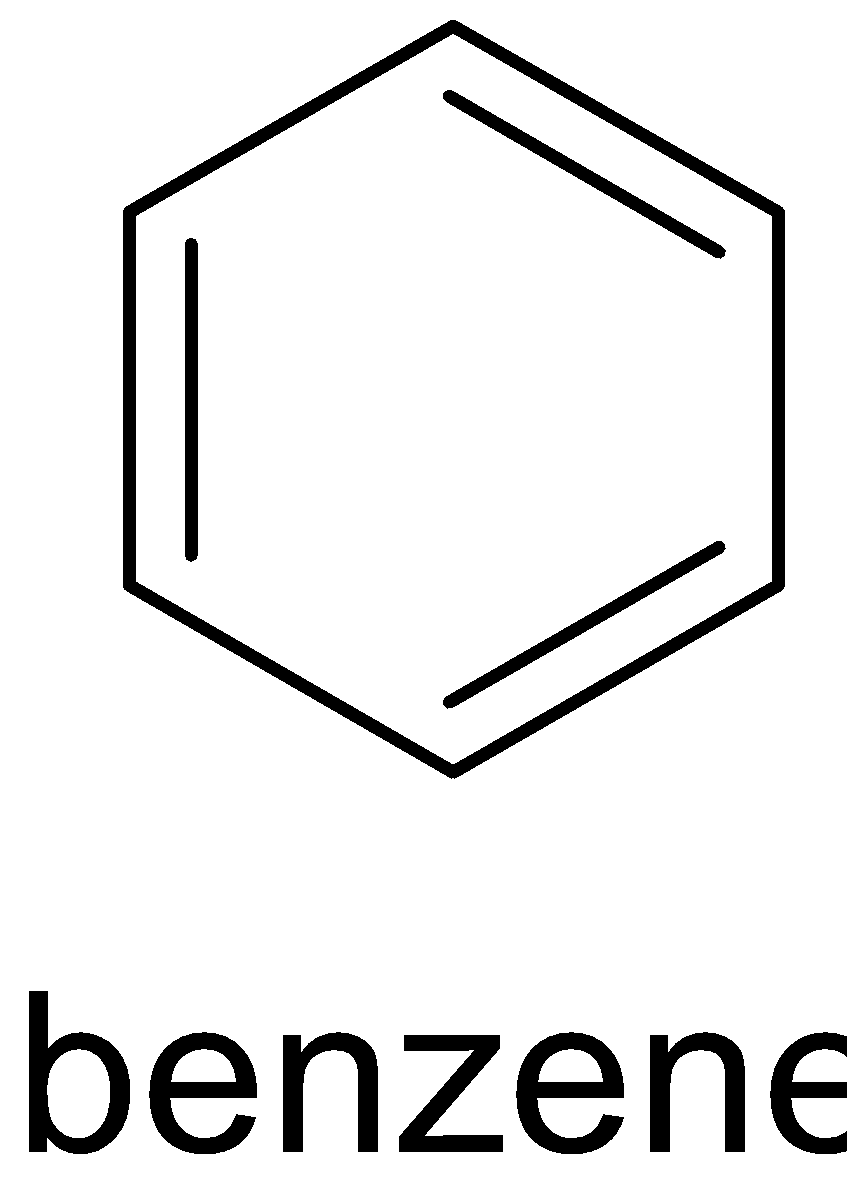
This reaction can only be done by heating phenol with zinc dust. The product formed will be benzene and zinc oxide. When phenol reacts with zinc dust, it releases proton which forms phenoxide ion. The proton then accepts an electron from zinc and produces a hydrogen radical and zinc cation. When phenoxide ion is heated, it forms phenyl radical. This phenyl radical reacts with the hydrogen radical and forms benzene. The complete reaction is given below:
${{\text{C}}_6}{{\text{H}}_5}{\text{OH}} \to {{\text{C}}_6}{{\text{H}}_5}{{\text{O}}^ - } + {{\text{H}}^ + }$
${{\text{H}}^ + } + {\text{Zn}} \to {{\text{H}}^ \bullet } + {\text{Z}}{{\text{n}}^ + }$
${{\text{C}}_6}{{\text{H}}_5}{{\text{O}}^ - }\xrightarrow{\Delta }{{\text{C}}_6}{{\text{H}}_5}^ \bullet + {{\text{O}}^{ \bullet - }}$
${{\text{C}}_6}{{\text{H}}_5}^ \bullet + {{\text{H}}^ \bullet } \to {{\text{C}}_6}{{\text{H}}_6}$
${\text{Z}}{{\text{n}}^ + } + {{\text{O}}^ - } \to {\text{Z}}{{\text{n}}^{2 + }} + {{\text{O}}^{2 - }} \to {\text{ZnO}}$
Zinc dust oxidizes itself to ${\text{ZnO}}$ and reduces phenol to benzene.
Therefore Y is zinc dust.
Soda lime is the mixture of ${\text{NaOH}}$ and ${\text{CaO}}$. Reaction of benzoic acid with soda lime is called Oakwood reaction. Sodium hydroxide removes ${\text{C}}{{\text{O}}_2}$ in benzoic acid molecules which produce alkane, i.e benzene. And the ${\text{C}}{{\text{O}}_2}$ reacts with sodium hydroxide to give sodium carbonate. The function of ${\text{CaO}}$ is that it makes ${\text{NaOH}}$ less reactive. The reaction is given below:
$$
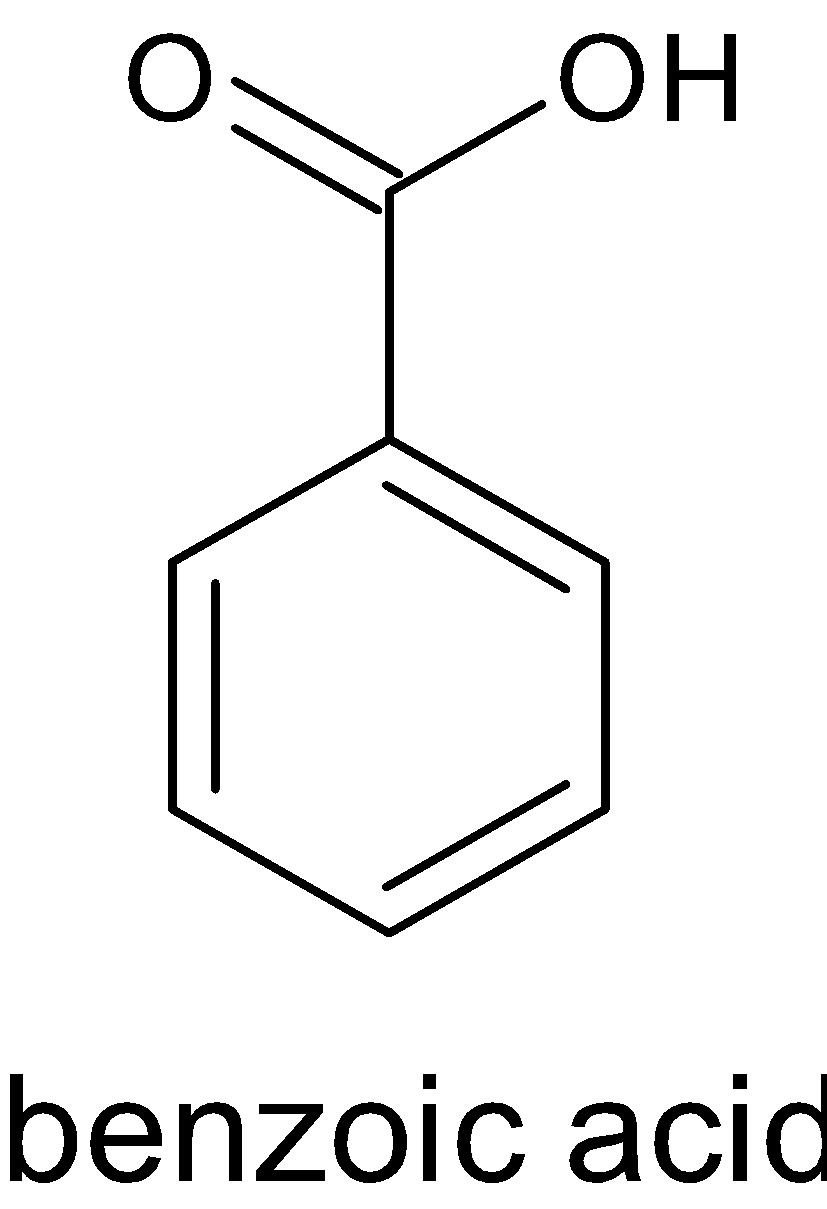 $ + {\text{NaOH}} + {\text{CaO}} \to $
$ + {\text{NaOH}} + {\text{CaO}} \to $
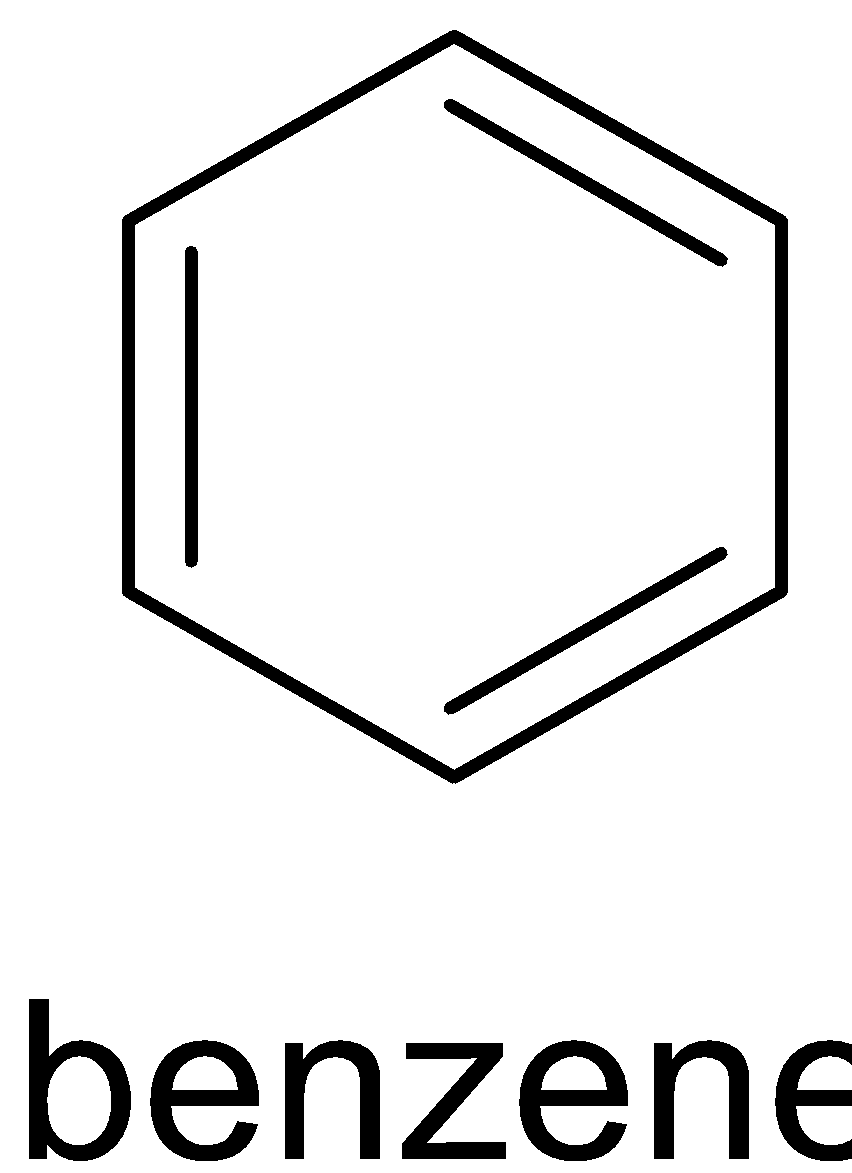 $ + {\text{N}}{{\text{a}}_2}{\text{C}}{{\text{O}}_3}$
$ + {\text{N}}{{\text{a}}_2}{\text{C}}{{\text{O}}_3}$
Hence, X is soda lime and Y is zinc dust.
So, the correct answer is option B.
Additional information Since ${\text{C}}{{\text{O}}_2}$ is removed, it is called a decarboxylation reaction. Soda lime is also used for decarboxylation of sodium salts of aromatic compounds. This reaction is called the Duma reaction.
Note:
At lower temperatures, when soda lime is used, benzoic acid forms sodium benzoate and then forms benzene and sodium carbonate. At higher temperatures, it directly forms benzene. This reaction is similar to pyrolysis.
Complete step by step answer:


This reaction can only be done by heating phenol with zinc dust. The product formed will be benzene and zinc oxide. When phenol reacts with zinc dust, it releases proton which forms phenoxide ion. The proton then accepts an electron from zinc and produces a hydrogen radical and zinc cation. When phenoxide ion is heated, it forms phenyl radical. This phenyl radical reacts with the hydrogen radical and forms benzene. The complete reaction is given below:
${{\text{C}}_6}{{\text{H}}_5}{\text{OH}} \to {{\text{C}}_6}{{\text{H}}_5}{{\text{O}}^ - } + {{\text{H}}^ + }$
${{\text{H}}^ + } + {\text{Zn}} \to {{\text{H}}^ \bullet } + {\text{Z}}{{\text{n}}^ + }$
${{\text{C}}_6}{{\text{H}}_5}{{\text{O}}^ - }\xrightarrow{\Delta }{{\text{C}}_6}{{\text{H}}_5}^ \bullet + {{\text{O}}^{ \bullet - }}$
${{\text{C}}_6}{{\text{H}}_5}^ \bullet + {{\text{H}}^ \bullet } \to {{\text{C}}_6}{{\text{H}}_6}$
${\text{Z}}{{\text{n}}^ + } + {{\text{O}}^ - } \to {\text{Z}}{{\text{n}}^{2 + }} + {{\text{O}}^{2 - }} \to {\text{ZnO}}$
Zinc dust oxidizes itself to ${\text{ZnO}}$ and reduces phenol to benzene.
Therefore Y is zinc dust.
Soda lime is the mixture of ${\text{NaOH}}$ and ${\text{CaO}}$. Reaction of benzoic acid with soda lime is called Oakwood reaction. Sodium hydroxide removes ${\text{C}}{{\text{O}}_2}$ in benzoic acid molecules which produce alkane, i.e benzene. And the ${\text{C}}{{\text{O}}_2}$ reacts with sodium hydroxide to give sodium carbonate. The function of ${\text{CaO}}$ is that it makes ${\text{NaOH}}$ less reactive. The reaction is given below:
$$


Hence, X is soda lime and Y is zinc dust.
So, the correct answer is option B.
Additional information Since ${\text{C}}{{\text{O}}_2}$ is removed, it is called a decarboxylation reaction. Soda lime is also used for decarboxylation of sodium salts of aromatic compounds. This reaction is called the Duma reaction.
Note:
At lower temperatures, when soda lime is used, benzoic acid forms sodium benzoate and then forms benzene and sodium carbonate. At higher temperatures, it directly forms benzene. This reaction is similar to pyrolysis.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




