
Benzene diazonium chloride on reaction with aniline in presence of dilute hydrochloric acid gives:
A)
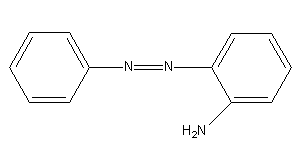
B)
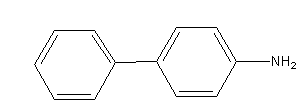
C)
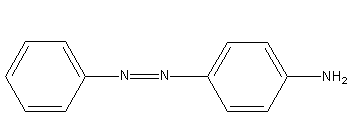
D)
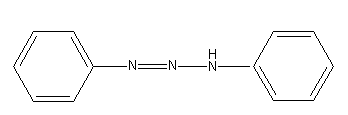




Answer
563.4k+ views
Hint: Benzene diazonium chloride is an organic compound having a formula\[{\text{Ar - }}{{\text{N}}_{\text{2}}}^ + {\text{C}}{{\text{l}}^{\text{ - }}}\]. It is intermediate in organic synthesis. Condensation of diazonium salts with aromatic amines in a dilute acidic solution to form azo dyes is known as coupling.
Complete solution:Aniline reacts with a mixture of sodium nitrite and hydrochloric acid and forms benzene diazonium chloride.The structure of Benzene diazonium chloride is
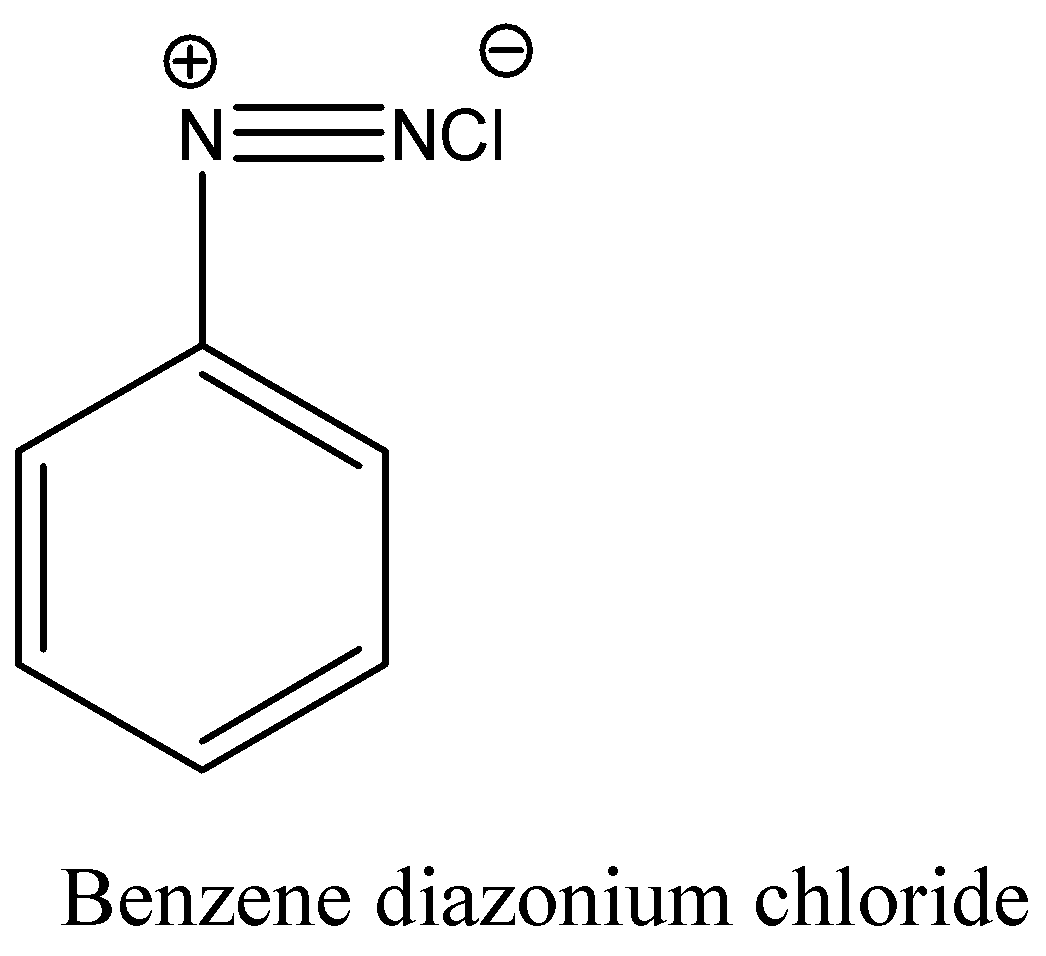
Benzene diazonium chloride reacts with aniline in presence of dilute hydrochloric acid and undergoes coupling reaction and produces stable azo products. In this reaction, benzene diazonium chloride acts as an electrophile in coupling with activated aniline. In this type of reaction, substitution takes place at the para position. So, the reaction Benzene diazonium chloride with aniline in presence of dilute hydrochloric acid is as follows:
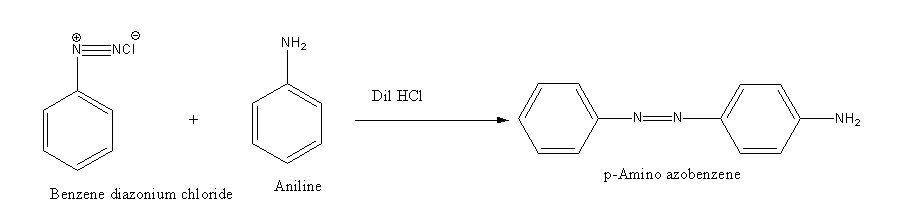
Thus, the correct options are (C).
Note: Aromatic diazonium ion is a very important intermediate to produce substituted benzene. The final product depends on reagent use. The reaction given to us is the azo coupling reaction. The azo coupling reaction is widely used to produce dyes, pigments, etc. In this type of reaction, substitution at para position is more favourable. If the para position is already occupied with substituent then substitution takes place at the ortho position.
Complete solution:Aniline reacts with a mixture of sodium nitrite and hydrochloric acid and forms benzene diazonium chloride.The structure of Benzene diazonium chloride is

Benzene diazonium chloride reacts with aniline in presence of dilute hydrochloric acid and undergoes coupling reaction and produces stable azo products. In this reaction, benzene diazonium chloride acts as an electrophile in coupling with activated aniline. In this type of reaction, substitution takes place at the para position. So, the reaction Benzene diazonium chloride with aniline in presence of dilute hydrochloric acid is as follows:

Thus, the correct options are (C).
Note: Aromatic diazonium ion is a very important intermediate to produce substituted benzene. The final product depends on reagent use. The reaction given to us is the azo coupling reaction. The azo coupling reaction is widely used to produce dyes, pigments, etc. In this type of reaction, substitution at para position is more favourable. If the para position is already occupied with substituent then substitution takes place at the ortho position.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




