
Benzoylation of phenol in alkaline medium is known as:
A. Friedel-Crafts reaction
B. Wurtz-Fittig reaction
C. Schotten-Baumann reaction
D. Sabatier Senderens reaction
Answer
605.7k+ views
Hint: The process given in the question is ‘benzoylation’. As the name suggests, benzoylation is the process of inserting a ‘benzoyl’ in any compound. Phenol is an alcohol and has one hydrogen it is capable of donating.
Complete step by step answer:
Schotten-Baumann reaction is a common method of synthesizing amides. It includes benzoylation of active hydrogen-containing compounds by using benzyl chloride and aqueous sodium hydroxide. The generalized reaction is given as followed –
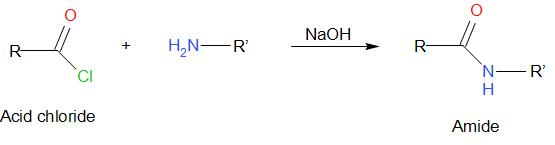
According to the question, we have
an active hydrogen containing compound – phenol
an alkaline medium – NaOH
and the objective is benzoylation of the compound. Therefore, we can conclude that benzoylation of phenol can be done by Schotten-Baumann reaction.
The reaction is as given below –
Therefore, the answer is – option (c) – Benzoylation of phenol in alkaline medium is known as Schotten-Baumann reaction.
Additional Information:
Benzoylation is a chemical reaction that introduces a benzoyl group into a molecule. Other bases can be used in this process instead of aq. NaOH, like pyridine.
Note: Let us see about the other reactions as well –
Sabatier Senderens reaction
It includes the reaction of hydrogen with carbon dioxide at temperatures around 300–400 degree Celsius and high pressures in the presence of a nickel catalyst to produce methane and water.
Friedel-Crafts reaction
It includes coupling reaction, which involves electrophilic aromatic substitution that is used for attachment of substituents to aromatic rings.
Wurtz-Fittig reaction
It includes chemical reaction of aryl halides with alkyl halides in presence of sodium metal and dry ether to give substituted aromatic compounds.
Complete step by step answer:
Schotten-Baumann reaction is a common method of synthesizing amides. It includes benzoylation of active hydrogen-containing compounds by using benzyl chloride and aqueous sodium hydroxide. The generalized reaction is given as followed –

According to the question, we have
an active hydrogen containing compound – phenol
an alkaline medium – NaOH
and the objective is benzoylation of the compound. Therefore, we can conclude that benzoylation of phenol can be done by Schotten-Baumann reaction.
The reaction is as given below –
Therefore, the answer is – option (c) – Benzoylation of phenol in alkaline medium is known as Schotten-Baumann reaction.
Additional Information:
Benzoylation is a chemical reaction that introduces a benzoyl group into a molecule. Other bases can be used in this process instead of aq. NaOH, like pyridine.
Note: Let us see about the other reactions as well –
Sabatier Senderens reaction
It includes the reaction of hydrogen with carbon dioxide at temperatures around 300–400 degree Celsius and high pressures in the presence of a nickel catalyst to produce methane and water.
Friedel-Crafts reaction
It includes coupling reaction, which involves electrophilic aromatic substitution that is used for attachment of substituents to aromatic rings.
Wurtz-Fittig reaction
It includes chemical reaction of aryl halides with alkyl halides in presence of sodium metal and dry ether to give substituted aromatic compounds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




