
Benzyl alcohol and sodium benzoate is obtained by the action of sodium hydroxide on benzaldehyde. This reaction is known as
A. Perkin reaction
B. Cannizzaro’s reaction
C. Sandmeyer’s reaction
D. Claisen reaction
Answer
233.1k+ views
Hint: We know that Cannizarro reaction is the disproportionation of two non-enolizable aldehydes in the presence of a base to give a primary alcohol and a carboxylic acid.
Complete step by step answer:
Benzyl alcohol and sodium benzoate is obtained by the reaction of benzaldehyde with sodium hydroxide. This is an example of Cannizzaro reaction. In this reaction, primary alcohol and sodium salt of carboxylic acid are obtained. The reaction can be shown as below:
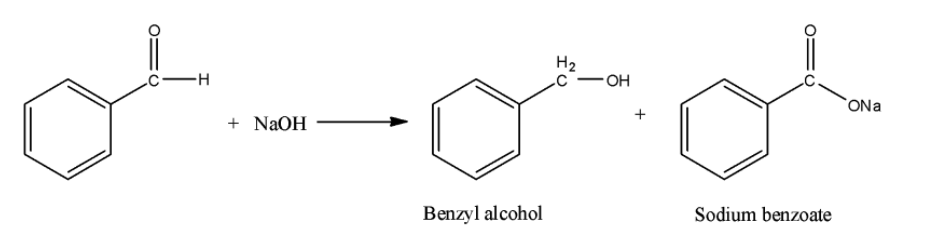
Hence, the correct option is B.
Additional Information
Let’s discuss the other three reactions given in options.
Option A is the Perkin reaction. In the Perkin reaction, an aromatic aldehyde and acid anhydride undergoes aldol condensation in presence of alkali salt of the acid to produce $\alpha ,\beta $-unsaturated aromatic acid.
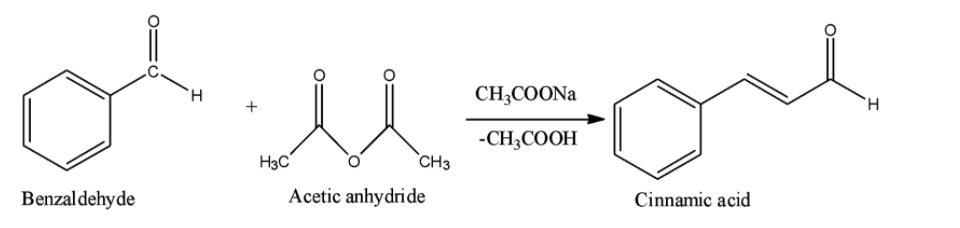
Option B is the Sandmeyer reaction. A chemical reaction that produces aryl halides from aryl diazonium salts using copper salts as catalyst is termed as Sandmeyer's reaction. This reaction is used in transformation of benzene like trifluoromethylation, cyanation etc.
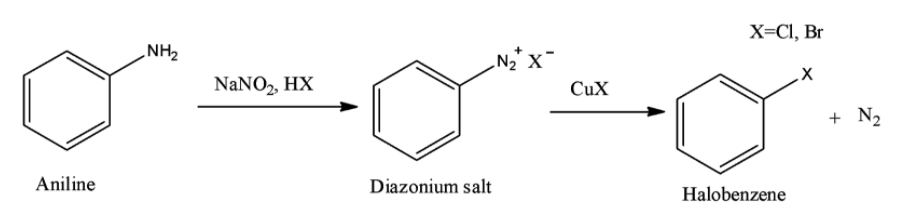
Option C is the Claisen reaction. The Claisen condensation is a reaction in which formation of carbon–carbon bond occurs between one ester and one carbonyl compound or between two esters in the presence of a strong base to form a $\beta $-keto ester or a $\beta $-diketone.

Note:
It is to be noted that Cannizzaro reaction is shown by only those aldehydes which do not have an alpha hydrogen atom. Alpha hydrogen is that hydrogen atom bonded to an alpha carbon.
Complete step by step answer:
Benzyl alcohol and sodium benzoate is obtained by the reaction of benzaldehyde with sodium hydroxide. This is an example of Cannizzaro reaction. In this reaction, primary alcohol and sodium salt of carboxylic acid are obtained. The reaction can be shown as below:

Hence, the correct option is B.
Additional Information
Let’s discuss the other three reactions given in options.
Option A is the Perkin reaction. In the Perkin reaction, an aromatic aldehyde and acid anhydride undergoes aldol condensation in presence of alkali salt of the acid to produce $\alpha ,\beta $-unsaturated aromatic acid.

Option B is the Sandmeyer reaction. A chemical reaction that produces aryl halides from aryl diazonium salts using copper salts as catalyst is termed as Sandmeyer's reaction. This reaction is used in transformation of benzene like trifluoromethylation, cyanation etc.

Option C is the Claisen reaction. The Claisen condensation is a reaction in which formation of carbon–carbon bond occurs between one ester and one carbonyl compound or between two esters in the presence of a strong base to form a $\beta $-keto ester or a $\beta $-diketone.

Note:
It is to be noted that Cannizzaro reaction is shown by only those aldehydes which do not have an alpha hydrogen atom. Alpha hydrogen is that hydrogen atom bonded to an alpha carbon.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




