
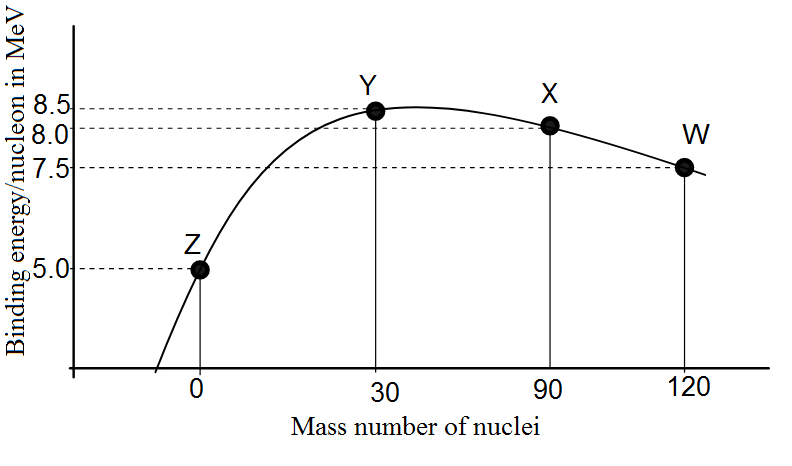
Binding energy per nucleon vs. mass number curve for nuclei is shown in fig. W, X, Y and Z are four nuclei indicated on the curve. The process that would release energy is
A. $Y \rightarrow 2Z$
B. $W \rightarrow X + Z$
C. $W \rightarrow 2Y$
D. $X \rightarrow Y+Z$

Answer
588.6k+ views
Hint: Binding energy per nucleon is a measure of stability of an atom. More the Binding energy per nucleon in a nuclei, more will be its stability. It is natural for an element to attain stability and reach a higher Binding energy per nucleon.
Complete answer:
We are familiar with ionization energy. In a hydrogen atom we require an energy of 13.6 eV to pull out its electron from the innermost orbit and make the hydrogen ionized. We can say that this energy is the binding energy of the electron to the nucleus (though we strictly use the term ionization energy).
In the case of a nucleus, binding energy is the amount of energy required to separate all the protons and neutrons constituting that nucleus. It can be also said that binding energy is the amount of energy given to bring all those protons and neutrons to make that nucleus. If we simply divide this binding energy by the amount of nucleons (protons + neutrons) we get binding energy per nucleon.
Therefore, we can get a sense that the more binding energy is, the more stable the system is.
Therefore, from the given curve, we require that we start with some element(s) that has low binding energy per nucleon (and hence low stability) and form an element(s) that has higher binding energy per nucleon.
Therefore, by comparing all the options in the light of this argument, we find that the correct answer is option (C). W breaks into 2Y nuclei.
Additional information:
All the elements that we know, can be divided into three categories,
1. light nuclei (He, O, etc) with low binding energy per nucleon.
2. Moderate nuclei with highest Binding energy per nucleon.
3. Heavy nuclei with low Binding energy per nucleon.
We know that two light nuclei with low Binding energies (per nucleon), combine to form an element with higher atomic number with higher Binding energy per nucleon. The process is nuclear fusion. Also, one heavy nucleus (unstable), creates two nuclei with higher binding energies (per nucleon). This process is called nuclear fission.
Note:
One might assume looking at the peak of the graph that the peak has an element with higher energy so it seems quite obvious that It breaks into elements with lower energies but that is not the case here. It is advisable to revise the definition of binding energy or to at least know the stability of an element by knowing its Binding energy.
Complete answer:
We are familiar with ionization energy. In a hydrogen atom we require an energy of 13.6 eV to pull out its electron from the innermost orbit and make the hydrogen ionized. We can say that this energy is the binding energy of the electron to the nucleus (though we strictly use the term ionization energy).
In the case of a nucleus, binding energy is the amount of energy required to separate all the protons and neutrons constituting that nucleus. It can be also said that binding energy is the amount of energy given to bring all those protons and neutrons to make that nucleus. If we simply divide this binding energy by the amount of nucleons (protons + neutrons) we get binding energy per nucleon.
Therefore, we can get a sense that the more binding energy is, the more stable the system is.
Therefore, from the given curve, we require that we start with some element(s) that has low binding energy per nucleon (and hence low stability) and form an element(s) that has higher binding energy per nucleon.
Therefore, by comparing all the options in the light of this argument, we find that the correct answer is option (C). W breaks into 2Y nuclei.
Additional information:
All the elements that we know, can be divided into three categories,
1. light nuclei (He, O, etc) with low binding energy per nucleon.
2. Moderate nuclei with highest Binding energy per nucleon.
3. Heavy nuclei with low Binding energy per nucleon.
We know that two light nuclei with low Binding energies (per nucleon), combine to form an element with higher atomic number with higher Binding energy per nucleon. The process is nuclear fusion. Also, one heavy nucleus (unstable), creates two nuclei with higher binding energies (per nucleon). This process is called nuclear fission.
Note:
One might assume looking at the peak of the graph that the peak has an element with higher energy so it seems quite obvious that It breaks into elements with lower energies but that is not the case here. It is advisable to revise the definition of binding energy or to at least know the stability of an element by knowing its Binding energy.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




