
What is the biuret test for urea? Explain with the chemical equation.
Answer
501.9k+ views
Hint: The biuret test is the test used for determining peptide bonds in any compound. It uses Biuret reagent which is one percent Copper (II) sulphate solution.
Complete answer:
The compound Biuret is formed by heating urea to about ${180^ \circ }C$. The $C{u^{ + 2}}$present in the Biuret forms a complex with the peptide bonds of the protein.
A peptide bond is formed when two molecules are by carbonyl and amino groups. The simplest of the proteins i.e., amino acids are attached via peptide bonds only. This test was discovered by Gustaw Piotrowski and is also known as the Piotrowski’s Test.
Initially the colour of the Copper sulphate solution will be Blue. When the Biuret in alkaline medium reacts with copper sulphate solution, it forms a copper chelate complex. This complex is of violet colour. Thus, we can say that in the presence of protein, the solution changes its colour from Blue to Violet.
As the number of peptide bonds increases the intensity of the violet colour also increases.
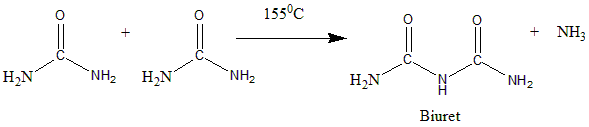
The chemical equation for biuret can be given as follows. In here two molecules of urea on heating releases ammonia gas and gives white crystalline solid of biuret.
Note:
This test is only useful for detecting the presence of Peptide bonds and is only given by Amino Acids and Histidine. Negative test is indicated by no change in colour. This test is the simplest way to detect the presence of peptide bonds and also the colour is stable. It only interacts with the Nitrogen of the protein, hence the Non-Protein Nitrogen doesn’t interfere.
Complete answer:
The compound Biuret is formed by heating urea to about ${180^ \circ }C$. The $C{u^{ + 2}}$present in the Biuret forms a complex with the peptide bonds of the protein.
A peptide bond is formed when two molecules are by carbonyl and amino groups. The simplest of the proteins i.e., amino acids are attached via peptide bonds only. This test was discovered by Gustaw Piotrowski and is also known as the Piotrowski’s Test.
Initially the colour of the Copper sulphate solution will be Blue. When the Biuret in alkaline medium reacts with copper sulphate solution, it forms a copper chelate complex. This complex is of violet colour. Thus, we can say that in the presence of protein, the solution changes its colour from Blue to Violet.
As the number of peptide bonds increases the intensity of the violet colour also increases.
The chemical equation for biuret can be given as follows. In here two molecules of urea on heating releases ammonia gas and gives white crystalline solid of biuret.

Note:
This test is only useful for detecting the presence of Peptide bonds and is only given by Amino Acids and Histidine. Negative test is indicated by no change in colour. This test is the simplest way to detect the presence of peptide bonds and also the colour is stable. It only interacts with the Nitrogen of the protein, hence the Non-Protein Nitrogen doesn’t interfere.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




