
Bond order of ‘B’ in $ B{F_3} $
(A) $ 1.0 $
(B) $ 3.0 $
(C) $ 1.33 $
(D) None
Answer
548.7k+ views
Hint: The number of the chemical bonds between the pair of atoms is indicated by the bond order. Bond order indicates the stability of a bond. We solve this sum by drawing the resonating structures and finding the bond order.
$ {\text{bond order}} = \dfrac{{{\text{Number of resonating bonds between any one bond of }}B{F_3}}}{{{\text{The total resonating structures of }}B{F_3}{\text{ }}}} $
Complete Step by step solution
Bond order is calculated by taking the ratio of the number of resonating bonds between any one bond and the total resonating structure.
Resonance helps us to describe bonding in certain molecules by the combination of several contributing structures. These contributing or resonating structures form resonance hybrid or hybrid structures.
The resonating structures of $ B{F_3} $ are as follows,
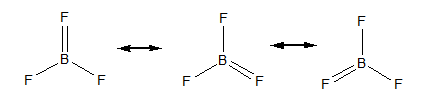
The total number of resonating structures of $ B{F_3} $ are three. To calculate the total number of resonating bonds, we will concentrate on any one B-F bond. Hence the total number of resonating bonds is equal to 4.
Bond order = $ \dfrac{{{\text{Number of resonating bonds between any one bond of }}B{F_3}}}{{{\text{The total resonating structures of }}B{F_3}{\text{ }}}} = \dfrac{4}{3} = 1.33 $
The bond order of $ B{F_3} $ is $ 1.33 $ .
Therefore, the correct answer is option C.
Note
$ B{F_3} $ is also known as boron trifluoride. It is a very useful lewis acid. As boron trifluoride is an important lewis acid, it is used as a reagent in organic synthesis. It is a pungent, colourless and toxic gas. It is used as a catalyst in isomerization, alkylation, esterification, dehydration, condensation, acylation and other reactions. Boron trifluoride initiates polymerization reaction of unsaturated compounds, particularly polyethers. Boron trifluoride is used to prepare diborane. Boron trifluoride is used as a flux in soldering of magnesium. Boron trifluoride is corrosive in nature. It is also used in fumigation
$ {\text{bond order}} = \dfrac{{{\text{Number of resonating bonds between any one bond of }}B{F_3}}}{{{\text{The total resonating structures of }}B{F_3}{\text{ }}}} $
Complete Step by step solution
Bond order is calculated by taking the ratio of the number of resonating bonds between any one bond and the total resonating structure.
Resonance helps us to describe bonding in certain molecules by the combination of several contributing structures. These contributing or resonating structures form resonance hybrid or hybrid structures.
The resonating structures of $ B{F_3} $ are as follows,

The total number of resonating structures of $ B{F_3} $ are three. To calculate the total number of resonating bonds, we will concentrate on any one B-F bond. Hence the total number of resonating bonds is equal to 4.
Bond order = $ \dfrac{{{\text{Number of resonating bonds between any one bond of }}B{F_3}}}{{{\text{The total resonating structures of }}B{F_3}{\text{ }}}} = \dfrac{4}{3} = 1.33 $
The bond order of $ B{F_3} $ is $ 1.33 $ .
Therefore, the correct answer is option C.
Note
$ B{F_3} $ is also known as boron trifluoride. It is a very useful lewis acid. As boron trifluoride is an important lewis acid, it is used as a reagent in organic synthesis. It is a pungent, colourless and toxic gas. It is used as a catalyst in isomerization, alkylation, esterification, dehydration, condensation, acylation and other reactions. Boron trifluoride initiates polymerization reaction of unsaturated compounds, particularly polyethers. Boron trifluoride is used to prepare diborane. Boron trifluoride is used as a flux in soldering of magnesium. Boron trifluoride is corrosive in nature. It is also used in fumigation
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




