
Bond order of N-O bond in \[NO_3^ - \] is:
A. 1
B. 2
C. 3
D. 1.33
Answer
565.2k+ views
Hint: In this question, the bond order of N-O in \[NO_3^ - \] is calculated by dividing the number of chemical bonds present between the atoms of the ion divided by the total number of resonating structures.
Complete step by step answer:
The nitrate ion \[NO_3^ - \], can be represented by more than one structure known as resonance structure. The resonance structure is defined as the set of Lewis structure which describes the delocalization of electrons in a polyatomic ion.
The resonance structure of nitrate ion is shown below.
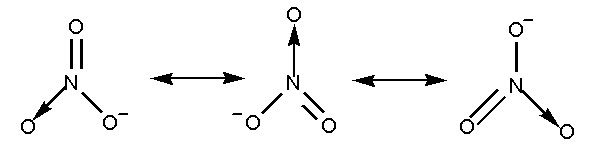
The nitrate ion contains three oxygen atoms attached to the nitrogen atom with a negative charge.
The bond order states the number of chemical bonds present between the atoms in a molecule. The bond order describes the stability of the bond.
For resonating structure, the bond order is calculated by the formula as shown below.
\[B.O = \dfrac{B}{R}\]
Where,
B.O is the bond order
B is total number of chemical bond present
R is the total number of resonating structure
In nitrate ion two oxygen atoms are attached by a single bond and one oxygen atom is attached by a double bond. Total 4 bonds are present.
Total 3 resonating structures are formed by the nitrate ion.
Substitute the values in the above equation.
\[ \Rightarrow B.O = \dfrac{4}{3}\]
\[ \Rightarrow B.O = 1.33\]
Thus, the bond order of N-O bonds in \[NO_3^ - \] is 1.33.
Therefore, the correct option is D.
Note:
The bond order is usually calculated using molecular orbital theory where the number of bonding electrons is subtracted by the number of antibonding electrons divided by 2.
Complete step by step answer:
The nitrate ion \[NO_3^ - \], can be represented by more than one structure known as resonance structure. The resonance structure is defined as the set of Lewis structure which describes the delocalization of electrons in a polyatomic ion.
The resonance structure of nitrate ion is shown below.

The nitrate ion contains three oxygen atoms attached to the nitrogen atom with a negative charge.
The bond order states the number of chemical bonds present between the atoms in a molecule. The bond order describes the stability of the bond.
For resonating structure, the bond order is calculated by the formula as shown below.
\[B.O = \dfrac{B}{R}\]
Where,
B.O is the bond order
B is total number of chemical bond present
R is the total number of resonating structure
In nitrate ion two oxygen atoms are attached by a single bond and one oxygen atom is attached by a double bond. Total 4 bonds are present.
Total 3 resonating structures are formed by the nitrate ion.
Substitute the values in the above equation.
\[ \Rightarrow B.O = \dfrac{4}{3}\]
\[ \Rightarrow B.O = 1.33\]
Thus, the bond order of N-O bonds in \[NO_3^ - \] is 1.33.
Therefore, the correct option is D.
Note:
The bond order is usually calculated using molecular orbital theory where the number of bonding electrons is subtracted by the number of antibonding electrons divided by 2.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




