
Bring out the following conversion.
Ethylamine to methylamine.
Answer
581.4k+ views
Hint: The conversion reaction requires the product having one carbon atom less than the reactant. This means that there will be a degradation of the reactant.
Complete step by step solution:
-Ethylamine has the molecular formula $C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}$. This is the compound having a primary aliphatic amine group. This compound exists in a gaseous state having ammonia-like odour. It can be converted to a liquid state just below room temperature. Ethylamine is a weak base.
-Methylamine is an organic compound having a molecular formula $C{{H}_{3}}N{{H}_{2}}$. This is the first homologue of the Amine family. Methylamine is used as the building block in many synthesis reactions. As it is an unhindered amine, it is a good nucleophile. This is also a weak base.
- Conversion of ethylamine to methylamine can take place as follows:
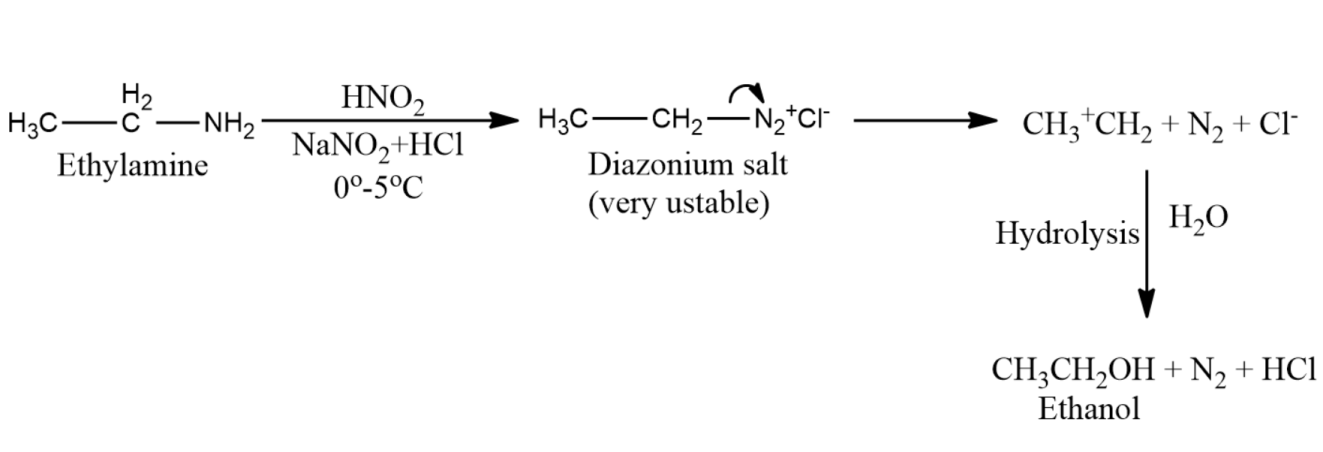
1) When ethylamine reacts with nitrous acid it gets converted to an ethyl diazonium salt. Diazonium salts are unstable. Therefore, ethyl diazonium salts get converted to ethyl alcohol thus liberating nitrogen gas and ethanol. Nitrous acid is prepared by acidifying the aqueous solution of sodium nitrate. Free nitrous acid is not stable and decomposes rapidly. This reaction is therefore done inside the reaction mixture which is known as in situ.
$\begin{align}
& C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}\xrightarrow{NaN{{O}_{2}}+HCl}[C{{H}_{3}}C{{H}_{2}}{{N}^{+}}C{{l}^{-}}] \\
& [C{{H}_{3}}C{{H}_{2}}{{N}^{+}}C{{l}^{-}}]\xrightarrow{{{H}_{2}}O}C{{H}_{3}}C{{H}_{2}}OH+{{N}_{2}}+HCl \\
\end{align}$
Mechanism:
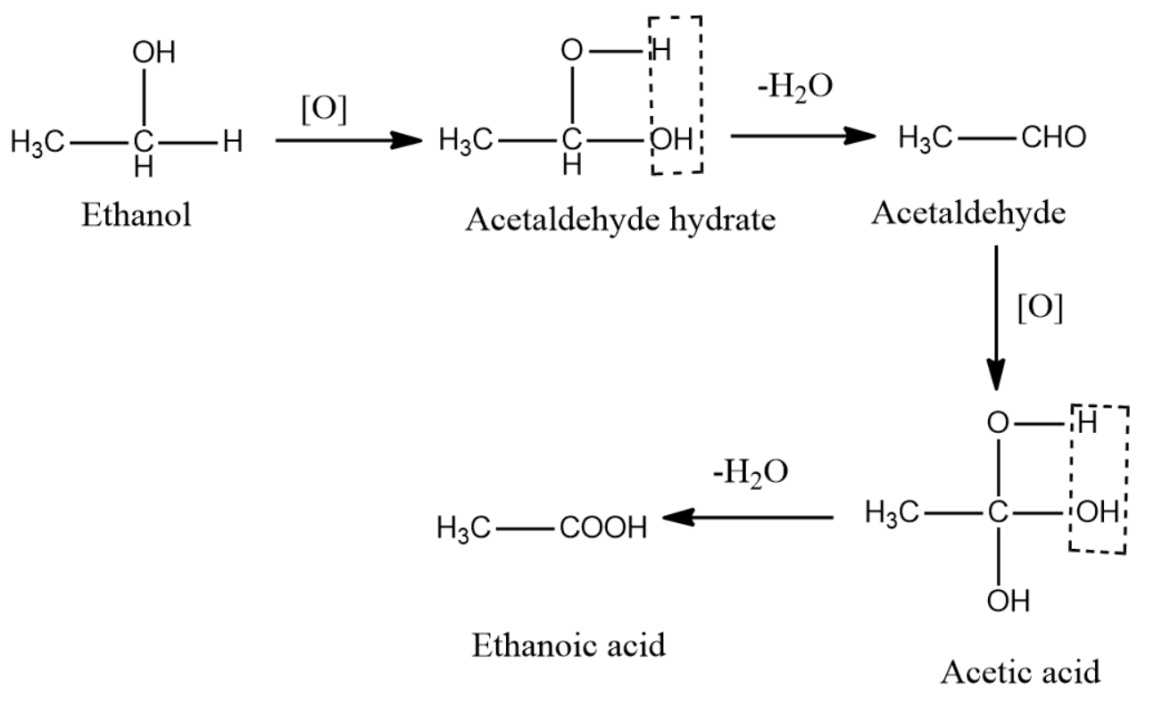
2) Now, ethanol can undergo oxidation on the addition of an oxidizing agent. Alcohol on oxidation first gets converted to Aldehyde which on further oxidation gives carboxylic acid.
So accordingly when ethanol reacts with an oxidizing agent it gives ethanoic acid.
$C{{H}_{2}}C{{H}_{2}}OH\xrightarrow{[O]}C{{H}_{3}}CHO\xrightarrow{[O[}C{{H}_{3}}COOH$
Mechanism:
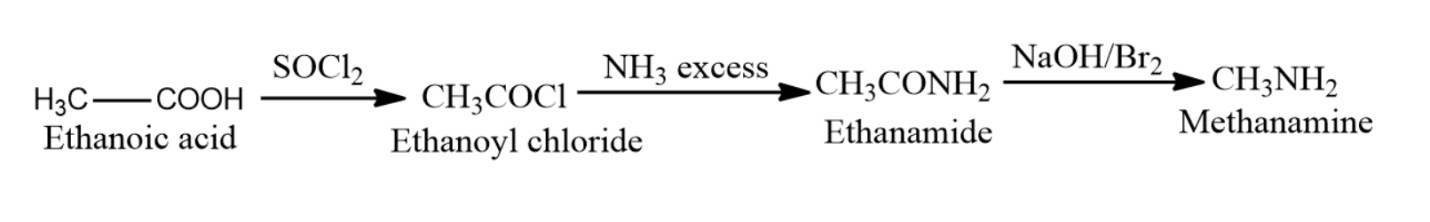
3) Ethanoic acid when heated in presence of ammonia then it gets converted to ethanamide. When an amide is treated with bromine in an aqueous solution of sodium hydroxide, then the amide undergoes a degradation reaction. The product thus formed will have one carbon atom less.
$C{{H}_{3}}COOH\xrightarrow[Heat]{N{{H}_{3}},SOC{{l}_{2}}}C{{H}_{3}}CON{{H}_{2}}$
$C{{H}_{3}}CON{{H}_{2}}\xrightarrow{NaOH/Br}C{{H}_{3}}N{{H}_{2}}$
Mechanism:
The final reaction can be given as follows:
$C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}+HN{{O}_{2}}\to C{{H}_{3}}C{{H}_{2}}OH\xrightarrow{[O]}C{{H}_{3}}COOH\xrightarrow{N{{H}_{3}}/SOC{{l}_{2}}}C{{H}_{3}}CON{{H}_{2}}\xrightarrow{NaOH/B{{r}_{2}}}C{{H}_{3}}N{{H}_{2}}$
Note: Ethanol can be oxidized by using only strong oxidizing agents. Oxidizing agents which can be used are potassium permanganate or potassium dichromate. Strong oxidants can convert primary alcohols to carboxylic acids, while weak oxidants convert primary oxidants to aldehydes. Secondary alcohols are oxidized to ketones by both oxidants, that is weak and strong. Tertiary alcohols are not at all oxidized.
Complete step by step solution:
-Ethylamine has the molecular formula $C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}$. This is the compound having a primary aliphatic amine group. This compound exists in a gaseous state having ammonia-like odour. It can be converted to a liquid state just below room temperature. Ethylamine is a weak base.
-Methylamine is an organic compound having a molecular formula $C{{H}_{3}}N{{H}_{2}}$. This is the first homologue of the Amine family. Methylamine is used as the building block in many synthesis reactions. As it is an unhindered amine, it is a good nucleophile. This is also a weak base.
- Conversion of ethylamine to methylamine can take place as follows:
1) When ethylamine reacts with nitrous acid it gets converted to an ethyl diazonium salt. Diazonium salts are unstable. Therefore, ethyl diazonium salts get converted to ethyl alcohol thus liberating nitrogen gas and ethanol. Nitrous acid is prepared by acidifying the aqueous solution of sodium nitrate. Free nitrous acid is not stable and decomposes rapidly. This reaction is therefore done inside the reaction mixture which is known as in situ.
$\begin{align}
& C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}\xrightarrow{NaN{{O}_{2}}+HCl}[C{{H}_{3}}C{{H}_{2}}{{N}^{+}}C{{l}^{-}}] \\
& [C{{H}_{3}}C{{H}_{2}}{{N}^{+}}C{{l}^{-}}]\xrightarrow{{{H}_{2}}O}C{{H}_{3}}C{{H}_{2}}OH+{{N}_{2}}+HCl \\
\end{align}$
Mechanism:

2) Now, ethanol can undergo oxidation on the addition of an oxidizing agent. Alcohol on oxidation first gets converted to Aldehyde which on further oxidation gives carboxylic acid.
So accordingly when ethanol reacts with an oxidizing agent it gives ethanoic acid.
$C{{H}_{2}}C{{H}_{2}}OH\xrightarrow{[O]}C{{H}_{3}}CHO\xrightarrow{[O[}C{{H}_{3}}COOH$
Mechanism:

3) Ethanoic acid when heated in presence of ammonia then it gets converted to ethanamide. When an amide is treated with bromine in an aqueous solution of sodium hydroxide, then the amide undergoes a degradation reaction. The product thus formed will have one carbon atom less.
$C{{H}_{3}}COOH\xrightarrow[Heat]{N{{H}_{3}},SOC{{l}_{2}}}C{{H}_{3}}CON{{H}_{2}}$
$C{{H}_{3}}CON{{H}_{2}}\xrightarrow{NaOH/Br}C{{H}_{3}}N{{H}_{2}}$
Mechanism:

The final reaction can be given as follows:
$C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}+HN{{O}_{2}}\to C{{H}_{3}}C{{H}_{2}}OH\xrightarrow{[O]}C{{H}_{3}}COOH\xrightarrow{N{{H}_{3}}/SOC{{l}_{2}}}C{{H}_{3}}CON{{H}_{2}}\xrightarrow{NaOH/B{{r}_{2}}}C{{H}_{3}}N{{H}_{2}}$
Note: Ethanol can be oxidized by using only strong oxidizing agents. Oxidizing agents which can be used are potassium permanganate or potassium dichromate. Strong oxidants can convert primary alcohols to carboxylic acids, while weak oxidants convert primary oxidants to aldehydes. Secondary alcohols are oxidized to ketones by both oxidants, that is weak and strong. Tertiary alcohols are not at all oxidized.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




