
Bromine water reacts with aniline to give:
A. O-bromoaniline
B. P-bromoaniline
C. m-bromoaniline
D. 2,4,6-tribromoaniline
Answer
544.5k+ views
Hint:Bromine water is a highly oxidising yellow to red mixture containing bromine dissolved in water. It is often used as a reactive chemical assay for the recognition of substances of substances that react with bromine. It results in the addition of bromide ions to the compound.
Complete step-by-step answer:Bromine water reacts with compounds that have unsaturated double and triple bonds. Other compounds that react with bromine water include phenol, enols, compounds with acetyl group, aniline, and glucose. The amino group in the aniline moiety is an ortho, para orienting group due to the lone of electrons on the nitrogen atom so when Bromine water reacts with aniline it gives 2,4,6-tribromoaniline, in which two bromo groups occupy the ortho positions at the 2nd and the 6th position while one of the bromo group occupies the para position at the 4th position.
The reaction can be given as follows:
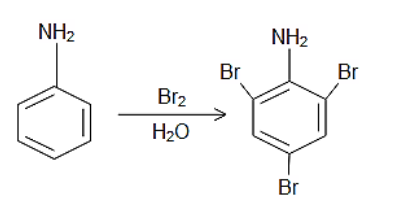
Thus, the correct answer is D.
Note: The ortho, para orienting groups are those that direct the incoming electrophile towards the 2nd, 4th, and the 6th positions of the phenyl ring while the meta-orienting groups direct the incoming electrophiles to either the 3rd or the 5th carbon atoms of the phenyl group. The ortho, para orienting groups are electron rings that help to stabilize the phenyl ring by going into extended conjugation with a ring using a double bond electron pair on the lone pair of electrons. The meta-orienting group on the other hand takes away the electrons from the aromatic ring mainly from the nearly ortho positions, hence the incoming electrophile is directed to the meta position.
Complete step-by-step answer:Bromine water reacts with compounds that have unsaturated double and triple bonds. Other compounds that react with bromine water include phenol, enols, compounds with acetyl group, aniline, and glucose. The amino group in the aniline moiety is an ortho, para orienting group due to the lone of electrons on the nitrogen atom so when Bromine water reacts with aniline it gives 2,4,6-tribromoaniline, in which two bromo groups occupy the ortho positions at the 2nd and the 6th position while one of the bromo group occupies the para position at the 4th position.
The reaction can be given as follows:

Thus, the correct answer is D.
Note: The ortho, para orienting groups are those that direct the incoming electrophile towards the 2nd, 4th, and the 6th positions of the phenyl ring while the meta-orienting groups direct the incoming electrophiles to either the 3rd or the 5th carbon atoms of the phenyl group. The ortho, para orienting groups are electron rings that help to stabilize the phenyl ring by going into extended conjugation with a ring using a double bond electron pair on the lone pair of electrons. The meta-orienting group on the other hand takes away the electrons from the aromatic ring mainly from the nearly ortho positions, hence the incoming electrophile is directed to the meta position.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




