
How is butanoic acid prepared starting from
i.An alcohol
ii.An alkyl halide
iii.An alkene
Answer
555.3k+ views
Hint: Butanoic acid is also known as butyric acid. Butanoic acid is a colourless clear liquid and has an unpleasant odour. The molecular formula for butanoic acid is ${\text{C}}{{\text{H}}_{\text{3}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{COOH}}$. We have to write the preparation reaction of butanoic acid from alcohol, alkyl halide and alkene.
Complete step-by-step answer:We have to write the preparation reaction of butanoic acid from alcohol, alkyl halide and alkene.
i.Butanoic acid can be prepared from an alcohol as follows:
Butanoic acid can be prepared from butanol which is an alcohol. Butanol reacts with acidified potassium dichromate and produces butanoic acid. The reaction is as follows:
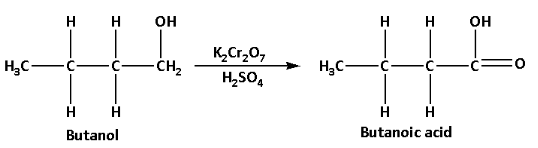
In this reaction, acidified potassium dichromate acts as an oxidising agent which oxidises butanol to butanoic acid.
ii.Butanoic acid can be prepared from an alkyl halide as follows:
Butanoic acid can be prepared from ethyl chloride which is an alkyl halide. Ethyl chloride reacts with sodium metal in presence of dry ether and produces butane. Butane on reaction with alcohol potassium permanganate produces butanoic acid. The reaction is as follows:
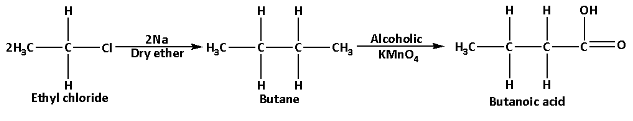
In this reaction, alcoholic potassium permanganate acts as an oxidising agent which oxidises butane to butanoic acid.
iii.Butanoic acid can be prepared from an alkene as follows:
Butanoic acid can be prepared from ethene which is an alkene. Ethene reacts with hydrochloric acid and produces ethyl chloride. Ethyl chloride reacts with sodium metal in presence of dry ether and produces butane. Butane in reaction with alcohol potassium permanganate produces butanoic acid. The reaction is as follows:
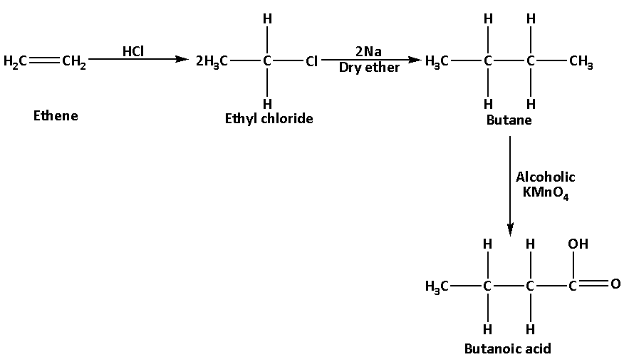
In this reaction, alcoholic potassium permanganate acts as an oxidising agent which oxidises butane to butanoic acid.
Note: Ethene reacts with hydrochloric acid according to the Markownikoff rule. The Markownikoff rule states that when an asymmetrical alkene reacts with an asymmetrical reagent, the negative part of the reagent gets attached to that carbon atom which has less number of hydrogen atoms.
Complete step-by-step answer:We have to write the preparation reaction of butanoic acid from alcohol, alkyl halide and alkene.
i.Butanoic acid can be prepared from an alcohol as follows:
Butanoic acid can be prepared from butanol which is an alcohol. Butanol reacts with acidified potassium dichromate and produces butanoic acid. The reaction is as follows:

In this reaction, acidified potassium dichromate acts as an oxidising agent which oxidises butanol to butanoic acid.
ii.Butanoic acid can be prepared from an alkyl halide as follows:
Butanoic acid can be prepared from ethyl chloride which is an alkyl halide. Ethyl chloride reacts with sodium metal in presence of dry ether and produces butane. Butane on reaction with alcohol potassium permanganate produces butanoic acid. The reaction is as follows:

In this reaction, alcoholic potassium permanganate acts as an oxidising agent which oxidises butane to butanoic acid.
iii.Butanoic acid can be prepared from an alkene as follows:
Butanoic acid can be prepared from ethene which is an alkene. Ethene reacts with hydrochloric acid and produces ethyl chloride. Ethyl chloride reacts with sodium metal in presence of dry ether and produces butane. Butane in reaction with alcohol potassium permanganate produces butanoic acid. The reaction is as follows:

In this reaction, alcoholic potassium permanganate acts as an oxidising agent which oxidises butane to butanoic acid.
Note: Ethene reacts with hydrochloric acid according to the Markownikoff rule. The Markownikoff rule states that when an asymmetrical alkene reacts with an asymmetrical reagent, the negative part of the reagent gets attached to that carbon atom which has less number of hydrogen atoms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




