
When calcium acetate and calcium formate together is subjected to dry distillation the product is:
A. Acetaldehyde
B. Acetone
C. Formaldehyde
D. None of these
Answer
571.5k+ views
Hint: Dry distillation is the process in which we heat solid materials to form gaseous products. It is also known as destructive distillation and is done with the help of wood or coal in a low concentration of oxygen and high temperature. It decomposes into several components which are separated in a fractional distillation column. In this process, usually organic matter is used which breaks down to gas or liquid and ultimately volatilizes (Part of such gas or liquid remains as a solid matter).
Complete step by step answer:
When calcium acetate undergoes dry distillation, it gives acetone as the main product. The equation for this reaction is:
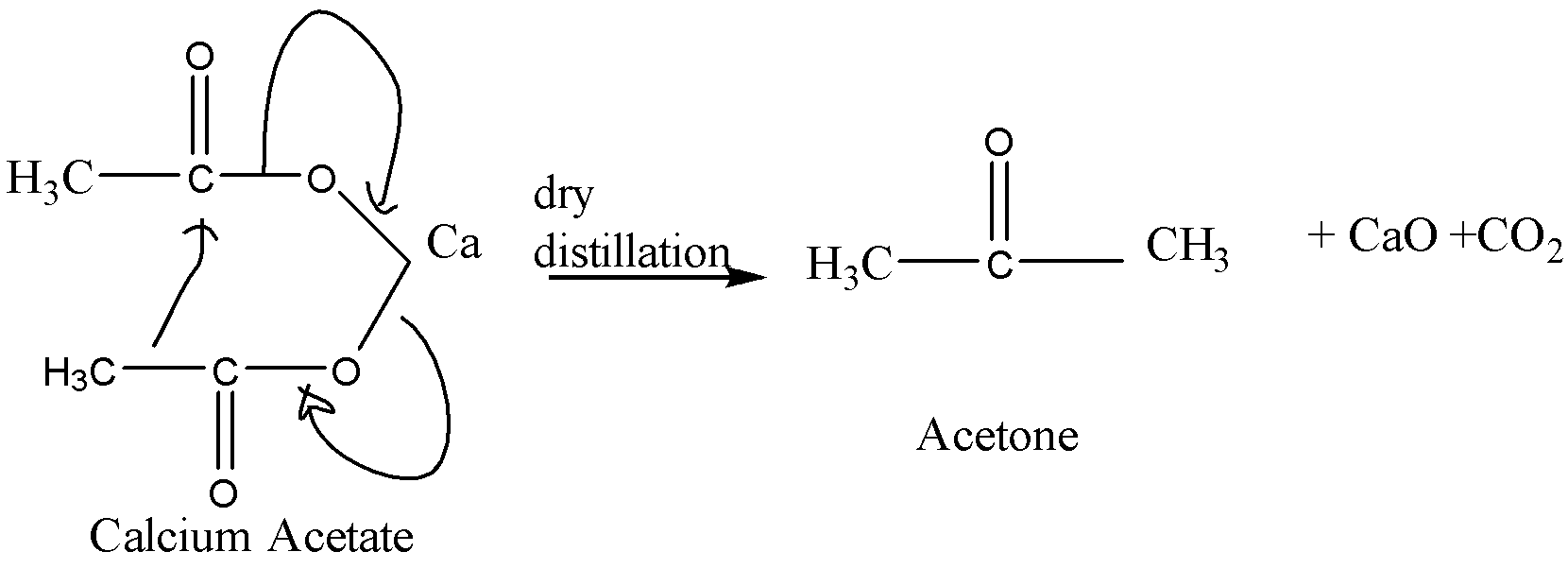
When calcium formate undergoes dry distillation, it gives formaldehyde as the main product. The equation for this reaction is:
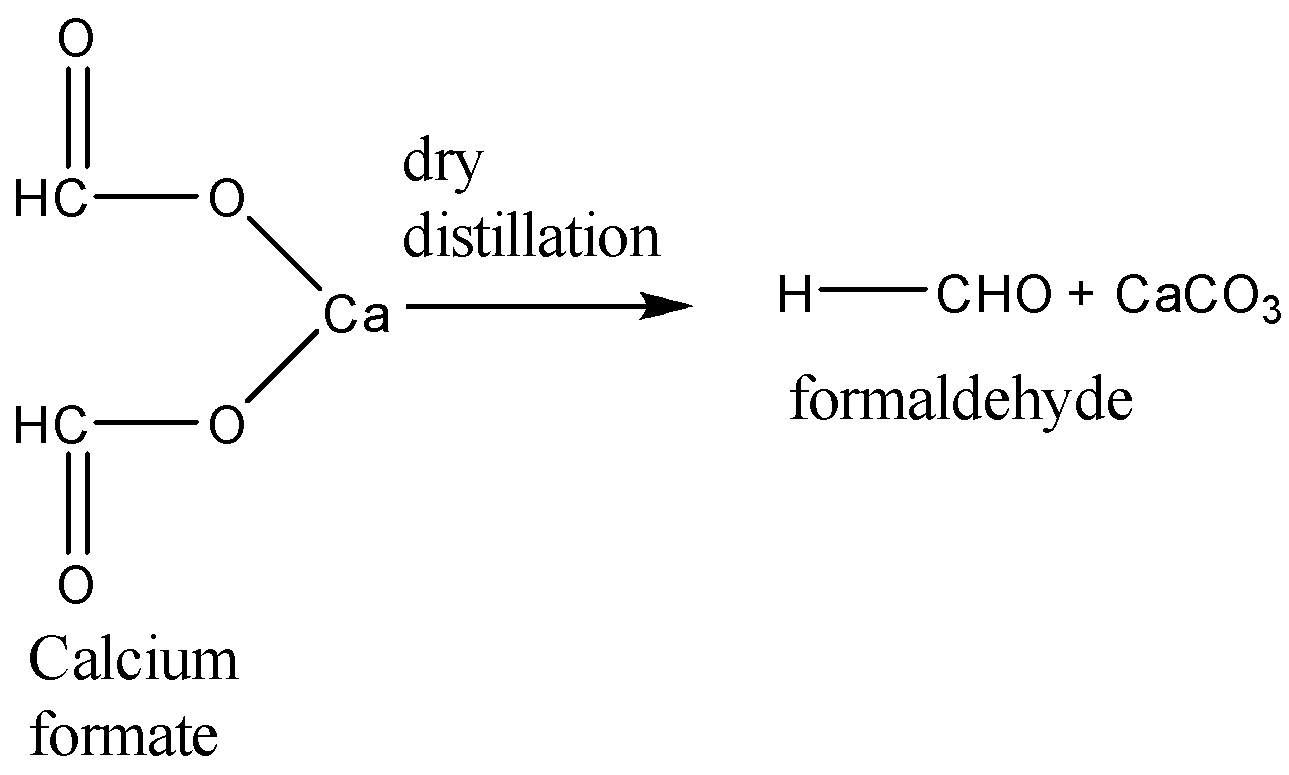
When calcium acetate and calcium formate are together subjected to dry distillation the reaction takes place as follows:
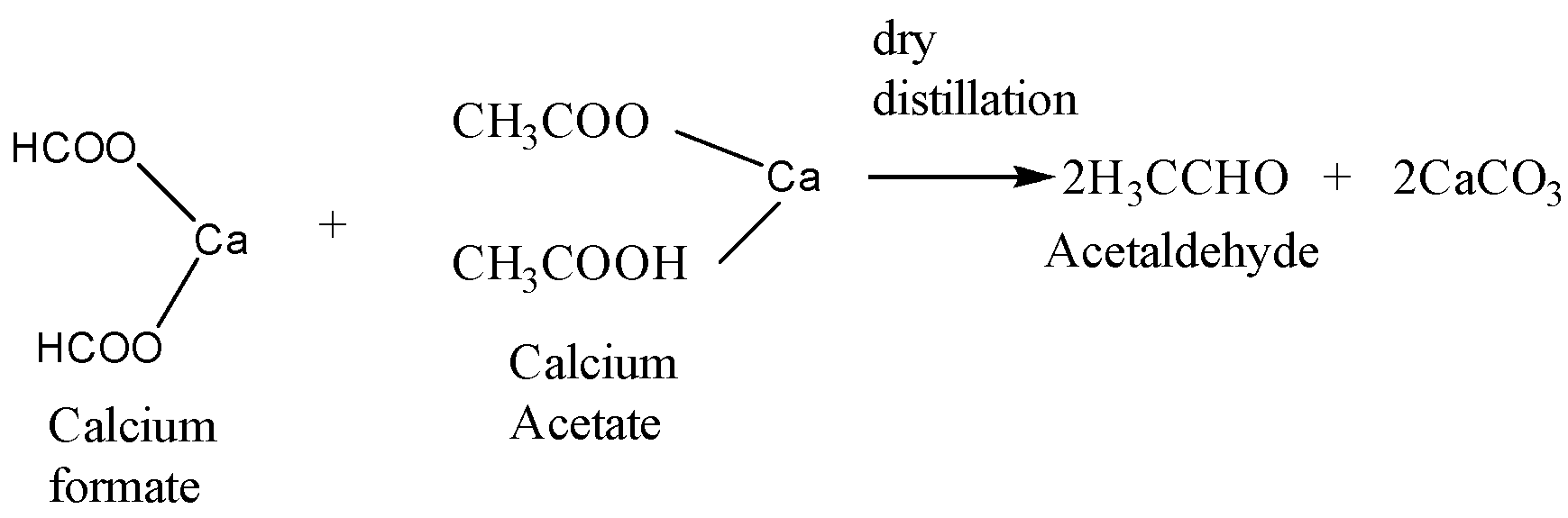
Calcium formate is the calcium salt of formic acid and calcium acetate is the calcium salt of acetic acid. When a mixture of these two undergoes dry distillation then acetaldehyde is formed as the major product and calcium carbonate is formed as the side product.
So, the correct answer is Option A .
Note: Mostly calcium salts of carboxylic acids give aldehydes and ketones.The process of dry distillation has been used to obtain liquid fuels from coal and wood and it can also be used to break down mineral salts such as sulphates for decades.Dry distillation may involve the processes of pyrolysis or thermolysis.Dry distillation in which chemical changes occur is a type of destructive distillation or it is also known as cracking.
Complete step by step answer:
When calcium acetate undergoes dry distillation, it gives acetone as the main product. The equation for this reaction is:

When calcium formate undergoes dry distillation, it gives formaldehyde as the main product. The equation for this reaction is:

When calcium acetate and calcium formate are together subjected to dry distillation the reaction takes place as follows:

Calcium formate is the calcium salt of formic acid and calcium acetate is the calcium salt of acetic acid. When a mixture of these two undergoes dry distillation then acetaldehyde is formed as the major product and calcium carbonate is formed as the side product.
So, the correct answer is Option A .
Note: Mostly calcium salts of carboxylic acids give aldehydes and ketones.The process of dry distillation has been used to obtain liquid fuels from coal and wood and it can also be used to break down mineral salts such as sulphates for decades.Dry distillation may involve the processes of pyrolysis or thermolysis.Dry distillation in which chemical changes occur is a type of destructive distillation or it is also known as cracking.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




