
Calcium hydroxide is used for making soap:
A. True
B. False
Answer
573.6k+ views
Hint: Calcium hydroxide is also known as slaked lime. It is white color amorphous solid and sparingly soluble in water. The suspension of the calcium hydroxide in water is known as milk of lime while the clean solution of it in water is known as lime water.
Complete step by step answer:
Soap is prepared by the saponification method. Since calcium ion is divalent if we used calcium ion instead of potassium or sodium ion for soap making then on reaction with a fatty acid, calcium ion produced an insoluble product so we can’t use calcium hydroxide for soap making.
Hence, the above state is false.
Additional information:
Soap is sodium and potassium salt of higher fatty acids such as lauric acid, palmitic acid, etc. The preparation of soap by saponification reaction involves the heating of fat or oil with an aqueous sodium hydroxide solution. The chemical equation for saponification reaction is given as:
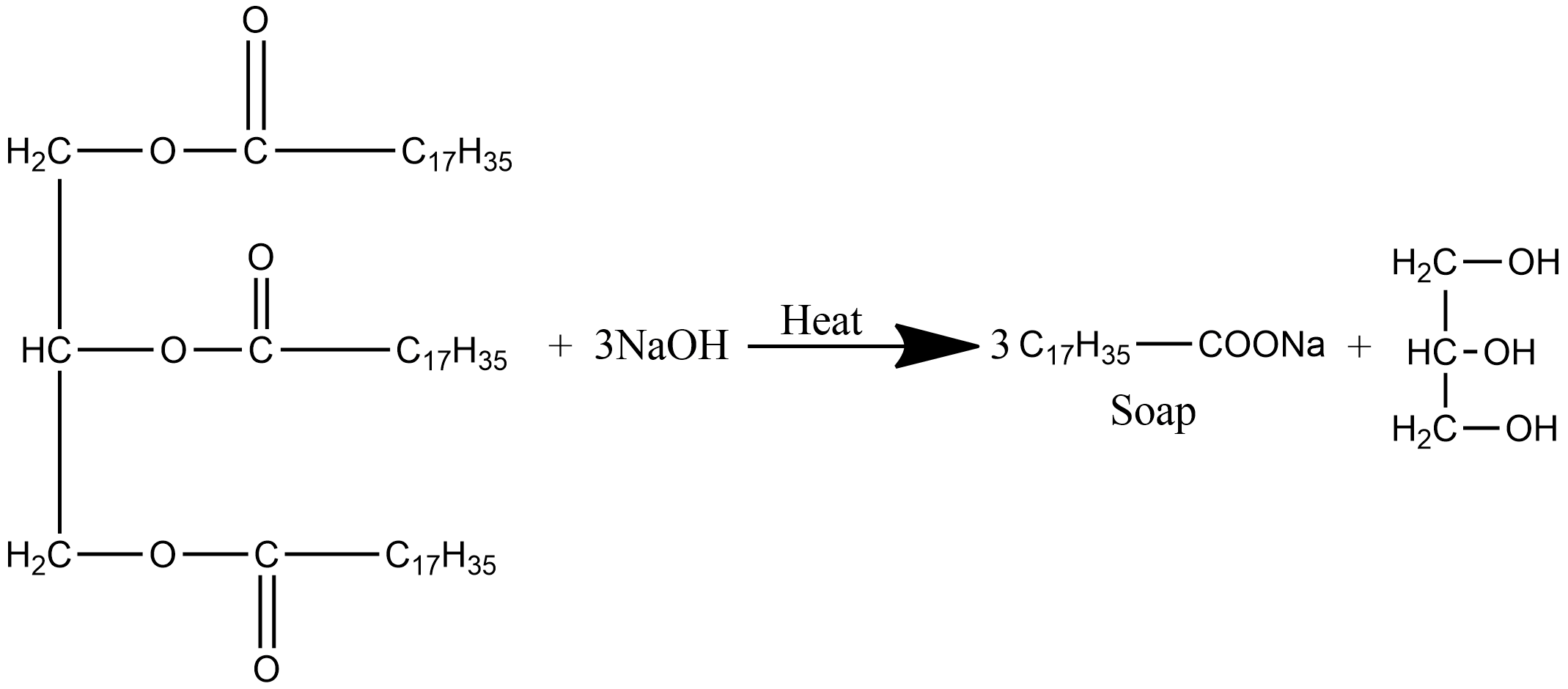
During the process of hydrolysis, the ester of fatty acids is hydrolyzed and the soap is obtained in the colloidal foam. It floats in the solution as curd and is precipitated from the solution by adding sodium chloride. The solution left after the removal of soap is called lye and it contains glycerol which can be obtained by fractional distillation. Out of sodium and potassium soap, potassium soap is generally soft to the skin.
Note: Soap is a good cleansing agent and is 100% biodegradable i.e., micro-organism present in sewage water can completely oxidize soap to carbon dioxide. As a result, it does not create any pollution problem.
Complete step by step answer:
Soap is prepared by the saponification method. Since calcium ion is divalent if we used calcium ion instead of potassium or sodium ion for soap making then on reaction with a fatty acid, calcium ion produced an insoluble product so we can’t use calcium hydroxide for soap making.
Hence, the above state is false.
Additional information:
Soap is sodium and potassium salt of higher fatty acids such as lauric acid, palmitic acid, etc. The preparation of soap by saponification reaction involves the heating of fat or oil with an aqueous sodium hydroxide solution. The chemical equation for saponification reaction is given as:

During the process of hydrolysis, the ester of fatty acids is hydrolyzed and the soap is obtained in the colloidal foam. It floats in the solution as curd and is precipitated from the solution by adding sodium chloride. The solution left after the removal of soap is called lye and it contains glycerol which can be obtained by fractional distillation. Out of sodium and potassium soap, potassium soap is generally soft to the skin.
Note: Soap is a good cleansing agent and is 100% biodegradable i.e., micro-organism present in sewage water can completely oxidize soap to carbon dioxide. As a result, it does not create any pollution problem.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




