
How do you calculate how many stereoisomers a compound has?
Answer
560.7k+ views
Hint: First, find the number of chiral carbon atoms in the compound, and then we can find the number of stereoisomers that the compound can show. ${{2}^{n}}$ can be used to find the number of stereoisomers where n is the number of chiral carbon atoms in the compound.
Complete step by step answer:
- Stereoisomers are those compounds having at least one chiral carbon atom, and there is a different arrangement of atoms in the space having the chemical formula same.
- We can define the chiral carbon atom as those carbon atoms in which all the substituents are different. These carbon atoms are represented with the star at the top. Only the carbon atoms having a single bond can be a chiral carbon atom, neither the double or triple bond having a carbon atom by a chiral carbon atom.
${{2}^{n}}$ can be used to find the number of stereoisomers where n is the number of chiral carbon atoms in the compound.
- For example, if the compound has one chiral carbon atom, then the value of n will be 1, and putting the value in the formula,
$={{2}^{n}}={{2}^{1}}=2$
Therefore, the compound will have 2 stereoisomers.
Let us take an example of 1,3-Dibromobutane, and see its structure is:
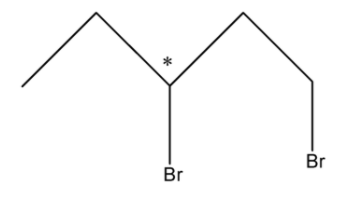
As we can see that in this compound there is only one chiral carbon atom in the compound.
So, there can be two Stereoisomers of 1, 3-Dibromopentane and these are drawn below:
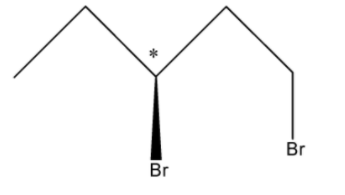
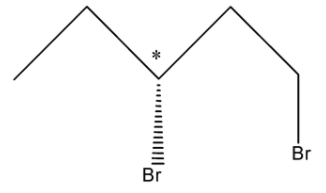
So, in the first structure, the bromine atom is above the plane and in the second structure, the bromine atom is below the plane.
Note: This formula is applicable only when the compound doesn’t have meso compounds i.e., there is internal symmetry in the molecule. Mostly meso compounds have two chiral carbon atoms. In this case, the number of meso compounds is subtracted from the ${{2}^{n}}$.
Complete step by step answer:
- Stereoisomers are those compounds having at least one chiral carbon atom, and there is a different arrangement of atoms in the space having the chemical formula same.
- We can define the chiral carbon atom as those carbon atoms in which all the substituents are different. These carbon atoms are represented with the star at the top. Only the carbon atoms having a single bond can be a chiral carbon atom, neither the double or triple bond having a carbon atom by a chiral carbon atom.
${{2}^{n}}$ can be used to find the number of stereoisomers where n is the number of chiral carbon atoms in the compound.
- For example, if the compound has one chiral carbon atom, then the value of n will be 1, and putting the value in the formula,
$={{2}^{n}}={{2}^{1}}=2$
Therefore, the compound will have 2 stereoisomers.
Let us take an example of 1,3-Dibromobutane, and see its structure is:

As we can see that in this compound there is only one chiral carbon atom in the compound.
So, there can be two Stereoisomers of 1, 3-Dibromopentane and these are drawn below:


So, in the first structure, the bromine atom is above the plane and in the second structure, the bromine atom is below the plane.
Note: This formula is applicable only when the compound doesn’t have meso compounds i.e., there is internal symmetry in the molecule. Mostly meso compounds have two chiral carbon atoms. In this case, the number of meso compounds is subtracted from the ${{2}^{n}}$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




