
Calculate the packing efficiency of a simple cubic cell?
Answer
570.3k+ views
Hint:
The packing efficiency can be defined as the ratio of the volume of a single atom in a unit cell to the volume of the entire unit cell multiplied by $100$ . It is a measure of the amount of volume that is occupied in a unit cell.
FORMULA USED: $PE = \dfrac{{\dfrac{4}{3}\pi {r^3}}}{{{a^3}}} \times 100$
Where $r$ is the radius of the atom and $a$ is the edge length of the simple cubic cell.
Complete answer:
The cubic unit cell is the simplest form of packing seen in a simple cubic structure.The packing efficiency of the simple cubic lattice can be found based on the length of the edge. The edge length can be derived as follows:
First, we have to consider the following image: we can see that the halves of two atoms lie along the edge of the cube. This means that if we consider the radius of each atom to be $r$ we can then concur that the length of the edge will be represented as follows,
$a = 2r$
Here $a$ is the edge length of the simple cubic lattice.
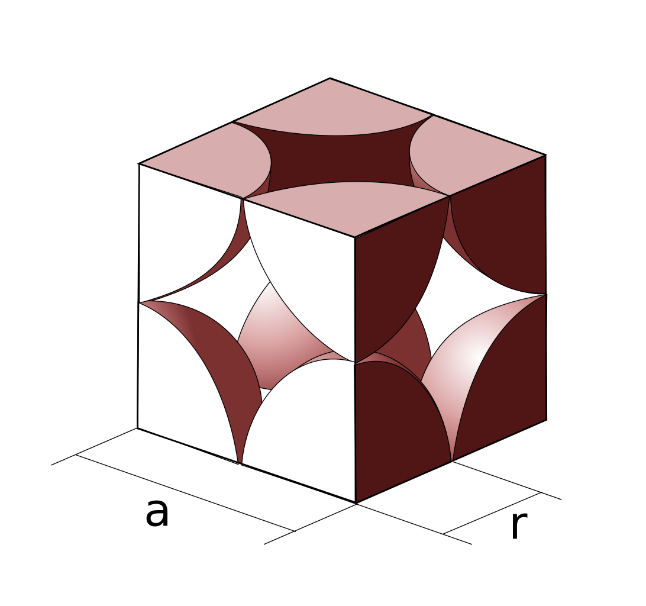
Using the formula mentioned above it will now be easy to derive the packing efficiency of this lattice. This is demonstrated below:
$PE = $ $\dfrac{{\dfrac{4}{3}\pi {r^3}}}{{{a^3}}}$$ \times 100$
we have to plug the value for edge length.
$ \Rightarrow \dfrac{{\dfrac{4}{3}\pi {r^3}}}{{{{\left( {2r} \right)}^3}}} \times 100$
Since $r$ is present both in the numerator and denominator, we can cancel that term to get the equation given below.
$ \Rightarrow \dfrac{{\dfrac{4}{3}\pi }}{{{{\left( 2 \right)}^3}}} \times 100$
$ \Rightarrow \dfrac{{4\pi }}{{3 \times 8}} \times 100$
cancelling the multiples of $4$ in the given equation, we now get,
$ \Rightarrow \dfrac{\pi }{{2 \times 3}} \times 100$
$ \Rightarrow 0.523 \times 100$
After multiplying with $100$ we get the percentage which is,
$ \Rightarrow 52.3\% $
Therefore, the packing efficiency of the simple cubic lattice is $52.3\% $ .
Note: The $52.3\% $ packing efficiency means that out of the $100\% $ of space or volume that is present in the lattice only $52.3\% $ of the lattice is occupied by the atoms and the remaining $47.7\% $ is made up of empty space also known as voids or interstitial spaces.
It is also imperative to remember the edge length of different crystal lattices as they differ as in face centered, body centered and others.
The packing efficiency can be defined as the ratio of the volume of a single atom in a unit cell to the volume of the entire unit cell multiplied by $100$ . It is a measure of the amount of volume that is occupied in a unit cell.
FORMULA USED: $PE = \dfrac{{\dfrac{4}{3}\pi {r^3}}}{{{a^3}}} \times 100$
Where $r$ is the radius of the atom and $a$ is the edge length of the simple cubic cell.
Complete answer:
The cubic unit cell is the simplest form of packing seen in a simple cubic structure.The packing efficiency of the simple cubic lattice can be found based on the length of the edge. The edge length can be derived as follows:
First, we have to consider the following image: we can see that the halves of two atoms lie along the edge of the cube. This means that if we consider the radius of each atom to be $r$ we can then concur that the length of the edge will be represented as follows,
$a = 2r$
Here $a$ is the edge length of the simple cubic lattice.

Using the formula mentioned above it will now be easy to derive the packing efficiency of this lattice. This is demonstrated below:
$PE = $ $\dfrac{{\dfrac{4}{3}\pi {r^3}}}{{{a^3}}}$$ \times 100$
we have to plug the value for edge length.
$ \Rightarrow \dfrac{{\dfrac{4}{3}\pi {r^3}}}{{{{\left( {2r} \right)}^3}}} \times 100$
Since $r$ is present both in the numerator and denominator, we can cancel that term to get the equation given below.
$ \Rightarrow \dfrac{{\dfrac{4}{3}\pi }}{{{{\left( 2 \right)}^3}}} \times 100$
$ \Rightarrow \dfrac{{4\pi }}{{3 \times 8}} \times 100$
cancelling the multiples of $4$ in the given equation, we now get,
$ \Rightarrow \dfrac{\pi }{{2 \times 3}} \times 100$
$ \Rightarrow 0.523 \times 100$
After multiplying with $100$ we get the percentage which is,
$ \Rightarrow 52.3\% $
Therefore, the packing efficiency of the simple cubic lattice is $52.3\% $ .
Note: The $52.3\% $ packing efficiency means that out of the $100\% $ of space or volume that is present in the lattice only $52.3\% $ of the lattice is occupied by the atoms and the remaining $47.7\% $ is made up of empty space also known as voids or interstitial spaces.
It is also imperative to remember the edge length of different crystal lattices as they differ as in face centered, body centered and others.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




