
Can alcohols hydrogen bond?
Answer
524.4k+ views
Hint: Alcohols are one of the organic compounds which are characterized by one or more hydroxyl groups which are further attached with carbon atoms in an alkyl group or hydrocarbon chain. Alcohols are considered derivatives of water.
Complete answer:
Hydrogen bond is defined as an attractive force between partially positive charged atoms with partially negative charged atoms. This is very weak in nature as compared to strength of covalent bond. Hydrogen bonds are usually shown as dotted lines between the atoms.
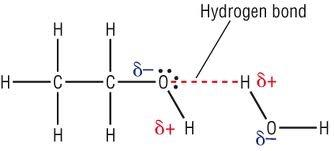
Alcohols can also hydrogen bond and we know that alcohol contains $OH$bonds and the formation of hydrogen bonding in alcohols can be explained due to the presence of very electronegative atom oxygen which shared the electron pair between oxygen and hydrogen are pulled towards the oxygen which is more electronegative in nature. This unequal distribution of electron pairs leads to the formation of two partial dipoles on both sides. The partially positive charged hydrogen is then attracted by the other partially negative charged oxygen and the phenomenon is known as hydrogen bonding. Hydrogen bonding is of two types generally known as intermolecular hydrogen bonding when bonding occurs between atoms of different molecules and intra molecular hydrogen bonding when bonding occurs between two partially charged atoms of same molecules.
Hence from the above discussion we can conclude that alcohols form hydrogen bonds and hydrogen bonding can be shown as:
Note:
Due to the presence of intermolecular hydrogen bonding in any polar compound the boiling point of that polar compound is higher as compared to non-polar compounds and also due to hydrogen bonding between organic compound and water the solubility of that compound also increases to some extent.
Complete answer:
Hydrogen bond is defined as an attractive force between partially positive charged atoms with partially negative charged atoms. This is very weak in nature as compared to strength of covalent bond. Hydrogen bonds are usually shown as dotted lines between the atoms.
Alcohols can also hydrogen bond and we know that alcohol contains $OH$bonds and the formation of hydrogen bonding in alcohols can be explained due to the presence of very electronegative atom oxygen which shared the electron pair between oxygen and hydrogen are pulled towards the oxygen which is more electronegative in nature. This unequal distribution of electron pairs leads to the formation of two partial dipoles on both sides. The partially positive charged hydrogen is then attracted by the other partially negative charged oxygen and the phenomenon is known as hydrogen bonding. Hydrogen bonding is of two types generally known as intermolecular hydrogen bonding when bonding occurs between atoms of different molecules and intra molecular hydrogen bonding when bonding occurs between two partially charged atoms of same molecules.
Hence from the above discussion we can conclude that alcohols form hydrogen bonds and hydrogen bonding can be shown as:

Note:
Due to the presence of intermolecular hydrogen bonding in any polar compound the boiling point of that polar compound is higher as compared to non-polar compounds and also due to hydrogen bonding between organic compound and water the solubility of that compound also increases to some extent.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




