
Cannizzaro’s reaction is not given by:
A.
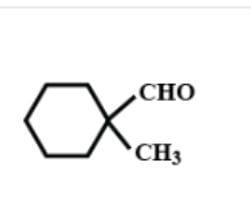
B.
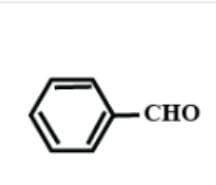
C.HCHO
D.$C{H_3}CHO$


Answer
583.2k+ views
Hint: Basically, Cannizzaro reaction is a chemical reaction that involves the base-induced disproportionation of two molecules of a non-enolizable aldehyde to yield a carboxylic acid and primary alcohol. Generally, in this reaction one molecule of alcohol and one molecule of carboxylic acid is obtained from two molecules of given aldehyde.
Complete step by step answer:
The Cannizzaro reaction was named after the scientist Stanislao Cannizzaro. It is basically a method to obtain one molecule of alcohol and one molecule of carboxylic acid from two molecules of given aldehyde. Now, let’s determine the mechanism of this reaction. Basically, a nucleophile such as hydroxide ion attacks the carbonyl group of the given aldehyde. This further leads to a disproportionation reaction and gives rise to an anion carrying two negative charges.
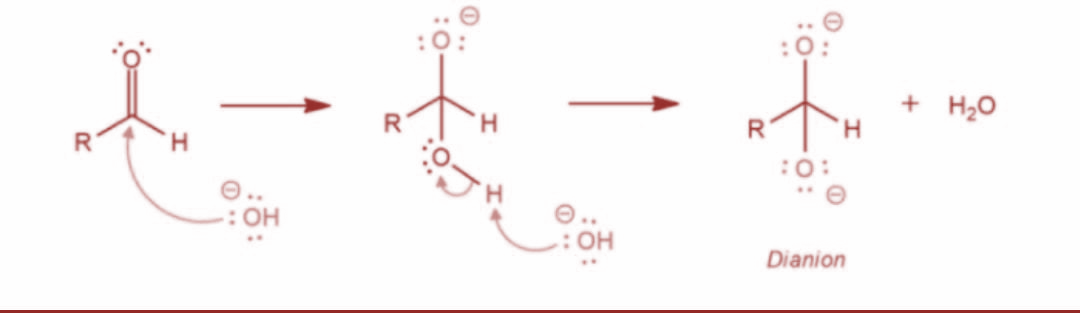
Now, in the next step the resulting intermediate functions as a hydride reducing agent. Further, the intermediate releases a hydride anion due to its unstable nature. The hydride anion proceeds to attack another aldehyde molecule and the doubly charged anion is converted into a carboxylate anion and aldehyde is converted into alkoxide ion. The reaction is as shown:
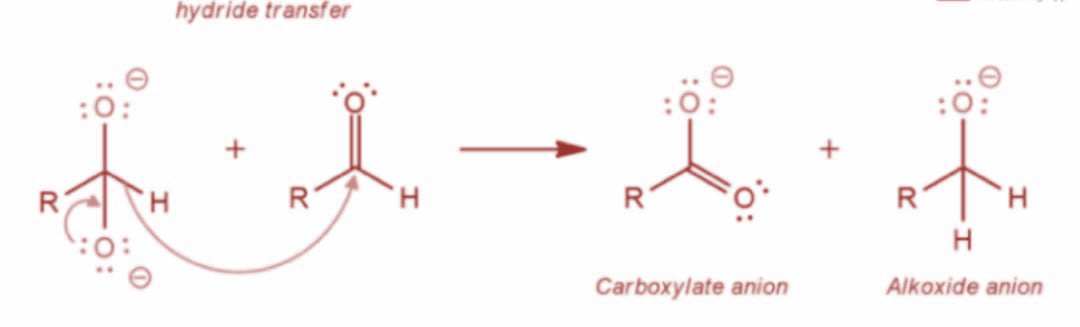
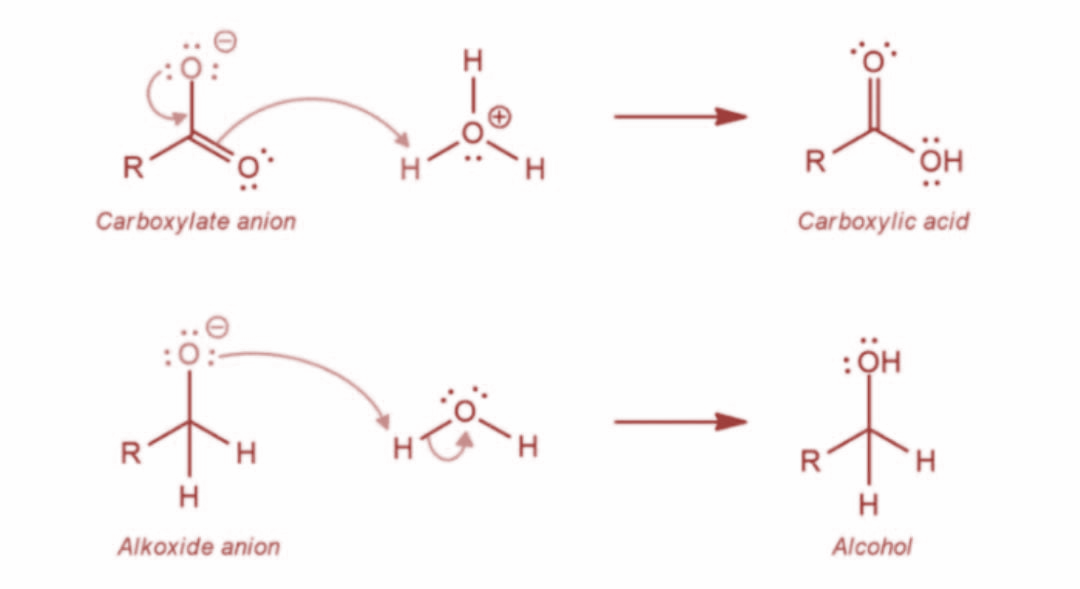
Now, in the last step, the final alcohol product is formed as water offers a proton to the alkoxide anion. Further, the carboxylate ion gives rise to the final carboxylic acid product. The reaction is as shown:
Now, acetaldehyde $(C{H_3}CHO)$ does not participate in this reaction because the alpha hydrogens are deprotonated due to the alkaline environment. Since acetaldehyde has three alpha hydrogens, it readily enolate ions upon deprotonation and hence cannot participate in the reaction.
Hence, option D is correct.
Note:Cannizzaro reaction is redox reaction. This is because one aldehyde is oxidized to give a carboxylic acid whereas the other aldehyde undergoes reduction to yield alcohol. Since both oxidation and reduction occurs in the hydride transfer so, this reaction is considered as a redox reaction.
Complete step by step answer:
The Cannizzaro reaction was named after the scientist Stanislao Cannizzaro. It is basically a method to obtain one molecule of alcohol and one molecule of carboxylic acid from two molecules of given aldehyde. Now, let’s determine the mechanism of this reaction. Basically, a nucleophile such as hydroxide ion attacks the carbonyl group of the given aldehyde. This further leads to a disproportionation reaction and gives rise to an anion carrying two negative charges.

Now, in the next step the resulting intermediate functions as a hydride reducing agent. Further, the intermediate releases a hydride anion due to its unstable nature. The hydride anion proceeds to attack another aldehyde molecule and the doubly charged anion is converted into a carboxylate anion and aldehyde is converted into alkoxide ion. The reaction is as shown:

Now, in the last step, the final alcohol product is formed as water offers a proton to the alkoxide anion. Further, the carboxylate ion gives rise to the final carboxylic acid product. The reaction is as shown:

Now, acetaldehyde $(C{H_3}CHO)$ does not participate in this reaction because the alpha hydrogens are deprotonated due to the alkaline environment. Since acetaldehyde has three alpha hydrogens, it readily enolate ions upon deprotonation and hence cannot participate in the reaction.
Hence, option D is correct.
Note:Cannizzaro reaction is redox reaction. This is because one aldehyde is oxidized to give a carboxylic acid whereas the other aldehyde undergoes reduction to yield alcohol. Since both oxidation and reduction occurs in the hydride transfer so, this reaction is considered as a redox reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




