
Caprolactum is the monomeric unit of
A) Nylon 6, 6
B) Nylon 2-Nylon-6
C) Nylon 6
D) None of these
Answer
565.5k+ views
Hint:The caprolactum is having the formulae $NH{{\left( C{{H}_{2}} \right)}_{5}}CO$
The polymers produced have a relation in the name with the monomers used in the polymeric process.
Complete answer:
So we have correctly identified the polymer which is synthesized from the monomer caprolactam.
Before moving to that part, let’s have a basic idea of polymers, types of polymers and the relation of the polymer name and the monomer used for its preparation.
Polymers are large molecules or macromolecules which are formed by the continuous addition of the monomeric units under suitable conditions. The process of formation of polymers from the monomeric units is called polymerization.
The polymers can be classified into many on the basis of parameters like-occurring of polymers, process involved in the polymerization, the nature of the monomers in the polymers, structure of the monomeric chains etc.
Classification on the basis of occurrence of polymer- natural polymer, synthetic polymer, semi-synthetic polymer.
Classification based on the process involved in polymerization-addition polymerization and condensation polymerization.
Classification based on the nature of monomers used- Homopolymer, Copolymer or heteropolymer.
Classification based on the structure of monomers chain-linear polymer, branched polymer and cross-linked polymer.
Now lets the monomer caprolactam, its molecular formulae is $NH{{\left( C{{H}_{2}} \right)}_{5}}CO$ or can be written as${{C}_{6}}{{H}_{11}}NO$ and the structure is,
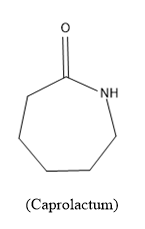
Caprolactum is a cyclohexane ring in which one carbon is replaced with –NH group and one carbon present is carbonyl carbon.
Caprolactum is the monomer used for synthesizing the polymer, Nylon-6.
In this production process the caprolactum is heated to a temperature of 553K for hours in an inert nitrogen atmosphere. During this polymerization process the caprolactam forms the polymer Nylon-6 through the ring opening reaction and forms a linear polymer chain.
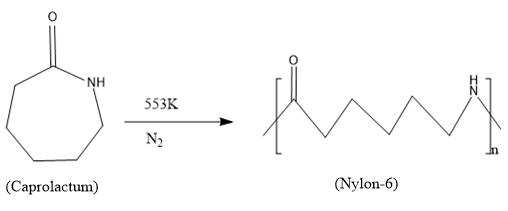
Since only one monomeric unit is involved it is a homopolymer and the digit 6 in nylon-6 represents that there are six carbon atoms in the monomeric structure.
Note:
The monomers used in nylon-6, 6 is adipic acid and hexamethylenediamine, both the monomers possess six carbon atoms in their structure hence there are two 6 digits in the polymer name.
The monomeric unit of nylon-2-nylon-6 is glycine and aminocaproic acid, in which the glycine has two carbon atoms and aminocaproic acid possesses six carbon atoms, hence the name.
The polymers produced have a relation in the name with the monomers used in the polymeric process.
Complete answer:
So we have correctly identified the polymer which is synthesized from the monomer caprolactam.
Before moving to that part, let’s have a basic idea of polymers, types of polymers and the relation of the polymer name and the monomer used for its preparation.
Polymers are large molecules or macromolecules which are formed by the continuous addition of the monomeric units under suitable conditions. The process of formation of polymers from the monomeric units is called polymerization.
The polymers can be classified into many on the basis of parameters like-occurring of polymers, process involved in the polymerization, the nature of the monomers in the polymers, structure of the monomeric chains etc.
Classification on the basis of occurrence of polymer- natural polymer, synthetic polymer, semi-synthetic polymer.
Classification based on the process involved in polymerization-addition polymerization and condensation polymerization.
Classification based on the nature of monomers used- Homopolymer, Copolymer or heteropolymer.
Classification based on the structure of monomers chain-linear polymer, branched polymer and cross-linked polymer.
Now lets the monomer caprolactam, its molecular formulae is $NH{{\left( C{{H}_{2}} \right)}_{5}}CO$ or can be written as${{C}_{6}}{{H}_{11}}NO$ and the structure is,

Caprolactum is a cyclohexane ring in which one carbon is replaced with –NH group and one carbon present is carbonyl carbon.
Caprolactum is the monomer used for synthesizing the polymer, Nylon-6.
In this production process the caprolactum is heated to a temperature of 553K for hours in an inert nitrogen atmosphere. During this polymerization process the caprolactam forms the polymer Nylon-6 through the ring opening reaction and forms a linear polymer chain.

Since only one monomeric unit is involved it is a homopolymer and the digit 6 in nylon-6 represents that there are six carbon atoms in the monomeric structure.
Note:
The monomers used in nylon-6, 6 is adipic acid and hexamethylenediamine, both the monomers possess six carbon atoms in their structure hence there are two 6 digits in the polymer name.
The monomeric unit of nylon-2-nylon-6 is glycine and aminocaproic acid, in which the glycine has two carbon atoms and aminocaproic acid possesses six carbon atoms, hence the name.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




