
When carbon and oxygen bond, the molecules contain how many pairs of bonding electrons?
Answer
552.9k+ views
Hint: The valence electrons are those electrons which take part in chemical bonding. The valence electrons in carbon is 4 and the valence electrons in oxygen is 6. The bonding electrons are those which form bonds.
Complete step by step answer:
There are many compounds where the carbon atom and oxygen atoms bond to form a compound. The number of carbon atoms and oxygen atoms can be more than one.
In carbon monoxide, the carbon atom is directly bonded to the oxygen atom. The molecular formula of carbon monoxide is CO. In carbon monoxide the carbon atom is bonded to oxygen atom by triple bond where one sigma bond is present and two pi bonds. The atomic number of carbon is 6 and the electronic configuration of carbon is $[He]2{s^2}2{p^2}$. The valence electrons in carbon is 4. The atomic number oxygen is 8 and the electronic configuration is $[He]2{s^2}2{p^4}$. The valence electrons in oxygen in 6. The total valence electrons in a carbon monoxide molecule is 10. For forming a triple bond between the carbon and oxygen atom, three electrons are shared by each atom. Total 6 valence electrons are used for bonding and are called bonding electrons and four valence electrons are the non-bonding electrons which are present as two electron pairs in carbon atom and oxygen atom.
The structure of carbon monoxide is shown below.
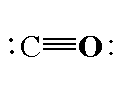
Note:
The other examples where the carbon and oxygen atoms are bonded is carbon dioxide, ethers which are represented as R-O-R where R is the alkyl group. In carbon dioxide the carbon atom is bonded with two oxygen with double bond. In ether the carbon is bonded with oxygen by double bond.
Complete step by step answer:
There are many compounds where the carbon atom and oxygen atoms bond to form a compound. The number of carbon atoms and oxygen atoms can be more than one.
In carbon monoxide, the carbon atom is directly bonded to the oxygen atom. The molecular formula of carbon monoxide is CO. In carbon monoxide the carbon atom is bonded to oxygen atom by triple bond where one sigma bond is present and two pi bonds. The atomic number of carbon is 6 and the electronic configuration of carbon is $[He]2{s^2}2{p^2}$. The valence electrons in carbon is 4. The atomic number oxygen is 8 and the electronic configuration is $[He]2{s^2}2{p^4}$. The valence electrons in oxygen in 6. The total valence electrons in a carbon monoxide molecule is 10. For forming a triple bond between the carbon and oxygen atom, three electrons are shared by each atom. Total 6 valence electrons are used for bonding and are called bonding electrons and four valence electrons are the non-bonding electrons which are present as two electron pairs in carbon atom and oxygen atom.
The structure of carbon monoxide is shown below.
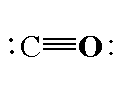
Note:
The other examples where the carbon and oxygen atoms are bonded is carbon dioxide, ethers which are represented as R-O-R where R is the alkyl group. In carbon dioxide the carbon atom is bonded with two oxygen with double bond. In ether the carbon is bonded with oxygen by double bond.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




