
Carboxylic acids can be prepared from alcohols, nitriles or esters. Which statements are correct?
1. Both primary and secondary alcohols can be oxidised to carboxylic acids.
2. Carboxylic acids can be made from nitriles by hydrolysis.
3. Ethyl propanoate gives propanoic acid when reacted by hydrochloric acid.
[A] 1, 2 and 3 are correct.
[B] 1 and 2 only are correct.
[C] 2 and 3 are correct.
[D] 1 is correct.
Answer
591k+ views
Hint: Carboxylic acids are compounds with –COOH functional group. Alcohols with 2 or more acidic hydrogen atoms present in them give us carboxylic acid on oxidation. Esters also give carboxylic acid in acidic medium.
Complete step by step answer:
We know that carboxylic acids are organic compounds and contain a –COOH group and are attached to a –R group where R refers to an alkyl group. The –COOH group is the carboxyl group and thus gives it the name carboxylic acid.
To answer this question, we will discuss each of the given statements one by one.
Firstly, we have that both primary and secondary alcohols can be oxidised to form carboxylic acid.
We know that alcohols are organic compounds that contain the –OH functional group which is the hydroxyl functional group.
In a primary alcohol, the carbon of the hydroxyl group is attached to only one alkyl group for example methanol, $C{{H}_{3}}OH$ (where R is hydrogen), ethanol, $C{{H}_{3}}C{{H}_{2}}OH$ where along with the hydroxyl group, the C-atom also contains a $C{{H}_{3}}$ group.
In secondary alcohols, the carbon containing the hydroxyl group is attached to two other alkyl groups for example 2-propanol, butan-2-ol etc.
We can oxidise primary alcohols to give us aldehydes and carboxylic acids but oxidation of secondary alcohol will give us ketone.
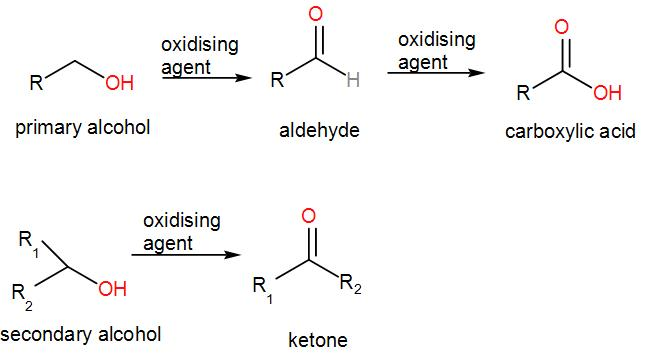
Therefore, the first statement is incorrect.
Next, we have Carboxylic acids that can be made from nitriles by hydrolysis.
We know that a nitrile is an organic compound with a –CN functional group. Hydrolysis of nitriles will give us carboxylic acid. In acidic or basic medium, the nitrile forms amide and then the amide is converted to carboxylic acid upon hydrolysis.
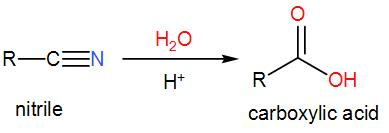
Therefore, the second statement is correct.
Lastly, we have Ethyl propanoate that gives propanoic acid when reacted by hydrochloric acid.
We know that ethyl propanoate is an ester and hydrolysis of ester in alcoholic medium gives us carboxylic acid and alcohol.
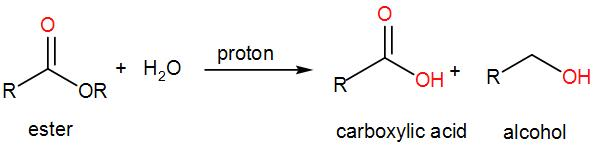
Therefore, the third statement is also correct.
As we can see from the above discussion that the second and the third statements are correct.
So, the correct answer is “Option C”.
Note: Carboxylic acids are formed from primary alcohols but not secondary alcohols because the hydrogen in the hydroxyl group is lost and also the hydrogen which is bonded to the secondary carbon is lost which causes the remaining oxygen to form double bond with carbon and thus gives us ketones instead of carboxylic acid. Whereas primary alcohols have at least 2 hydrogen atoms attached with the carbon thus oxidation causes formation of carboxylic acid.
Complete step by step answer:
We know that carboxylic acids are organic compounds and contain a –COOH group and are attached to a –R group where R refers to an alkyl group. The –COOH group is the carboxyl group and thus gives it the name carboxylic acid.
To answer this question, we will discuss each of the given statements one by one.
Firstly, we have that both primary and secondary alcohols can be oxidised to form carboxylic acid.
We know that alcohols are organic compounds that contain the –OH functional group which is the hydroxyl functional group.
In a primary alcohol, the carbon of the hydroxyl group is attached to only one alkyl group for example methanol, $C{{H}_{3}}OH$ (where R is hydrogen), ethanol, $C{{H}_{3}}C{{H}_{2}}OH$ where along with the hydroxyl group, the C-atom also contains a $C{{H}_{3}}$ group.
In secondary alcohols, the carbon containing the hydroxyl group is attached to two other alkyl groups for example 2-propanol, butan-2-ol etc.
We can oxidise primary alcohols to give us aldehydes and carboxylic acids but oxidation of secondary alcohol will give us ketone.

Therefore, the first statement is incorrect.
Next, we have Carboxylic acids that can be made from nitriles by hydrolysis.
We know that a nitrile is an organic compound with a –CN functional group. Hydrolysis of nitriles will give us carboxylic acid. In acidic or basic medium, the nitrile forms amide and then the amide is converted to carboxylic acid upon hydrolysis.

Therefore, the second statement is correct.
Lastly, we have Ethyl propanoate that gives propanoic acid when reacted by hydrochloric acid.
We know that ethyl propanoate is an ester and hydrolysis of ester in alcoholic medium gives us carboxylic acid and alcohol.

Therefore, the third statement is also correct.
As we can see from the above discussion that the second and the third statements are correct.
So, the correct answer is “Option C”.
Note: Carboxylic acids are formed from primary alcohols but not secondary alcohols because the hydrogen in the hydroxyl group is lost and also the hydrogen which is bonded to the secondary carbon is lost which causes the remaining oxygen to form double bond with carbon and thus gives us ketones instead of carboxylic acid. Whereas primary alcohols have at least 2 hydrogen atoms attached with the carbon thus oxidation causes formation of carboxylic acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




