
How many C-atoms are there in a pyranose ring?
A) 3
B) 5
C) 6
D) 7
Answer
591k+ views
Hint: The sugars can be written using the Haworth’s formula. Pyranose and Furanose are the oxides rings that resemble the oxygen containing heterocyclic ring pyran and furan respectively. These are five or six-membered rings where one of the positions is occupied by oxide.
Complete step by step answer:
Pyranose is a term used for the saccharides that have the chemical structure. It is a chemical structure for the six-membered rings which consist of five carbon atoms and one oxygen atom in it. There can be other external carbons to the ring.
The structure of $\text{ }\!\!\alpha\!\!\text{ -D-glucose}$ and $\text{ }\!\!\beta\!\!\text{ -D-glucose}$ may be drawn in a simple six-membered ring which is called pyranose structures. These structures resemble the pyran which is a six-membered heterocyclic ring containing five carbon atoms and one oxygen atom.
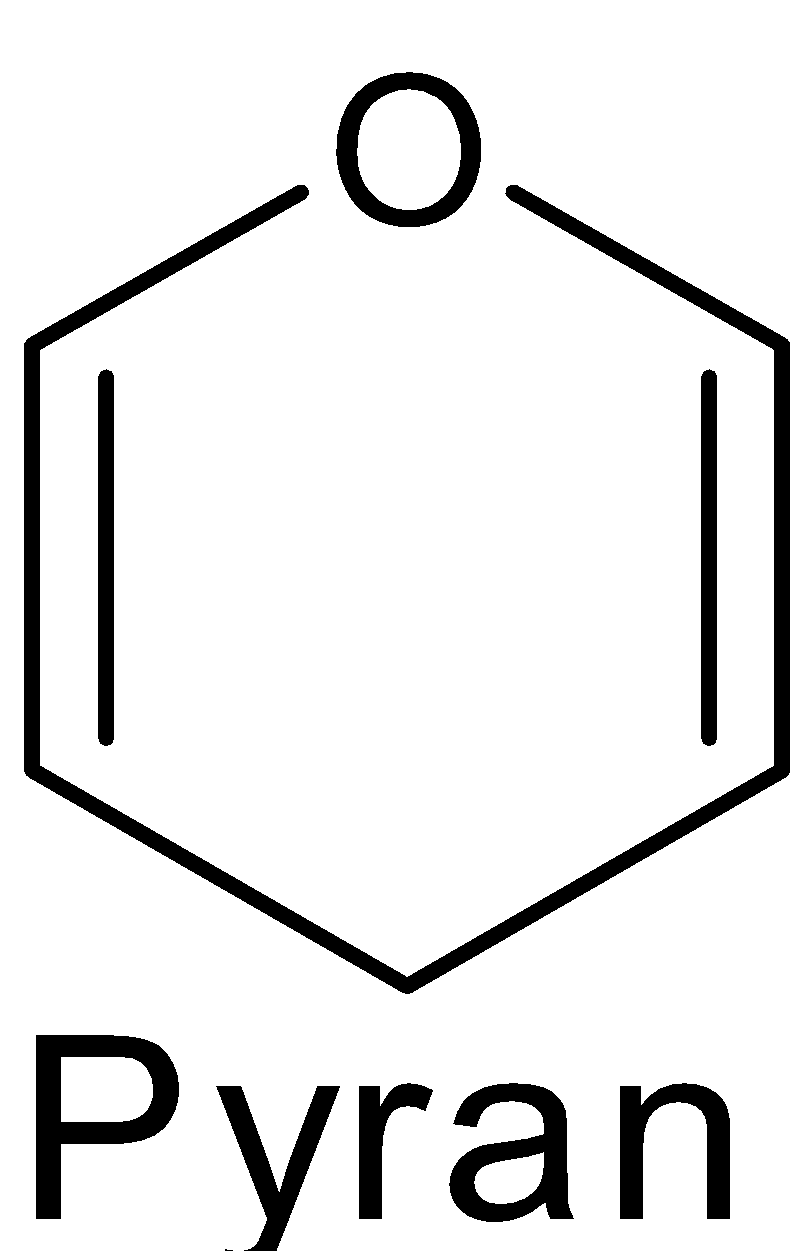
These structures were suggested by Haworth and are known as the Haworth projection formulae or pyranose structures.
To write the pyranose structures for any monosaccharide$\text{( }\!\!\alpha\!\!\text{ -and }\!\!\beta\!\!\text{ -D-glucose)}$, first draw a hexagon with its oxygen atom at the upper right-hand corner. The terminal $\text{-C}{{\text{H}}_{\text{2}}}\text{OH}$ group as shown in the figure is always placed above the plane of the hexagon ring. Place all the groups which are present on the left-hand side in Fischer projection above the plane of the ring and all those groups on the right hand in Fischer projection below the plane of the ring.
Pyranose rings are formed by the reaction of the hydroxyl group on the carbon number five of sugar with the aldehyde which is at the carbon one. This formation goes through the hemiacetal.
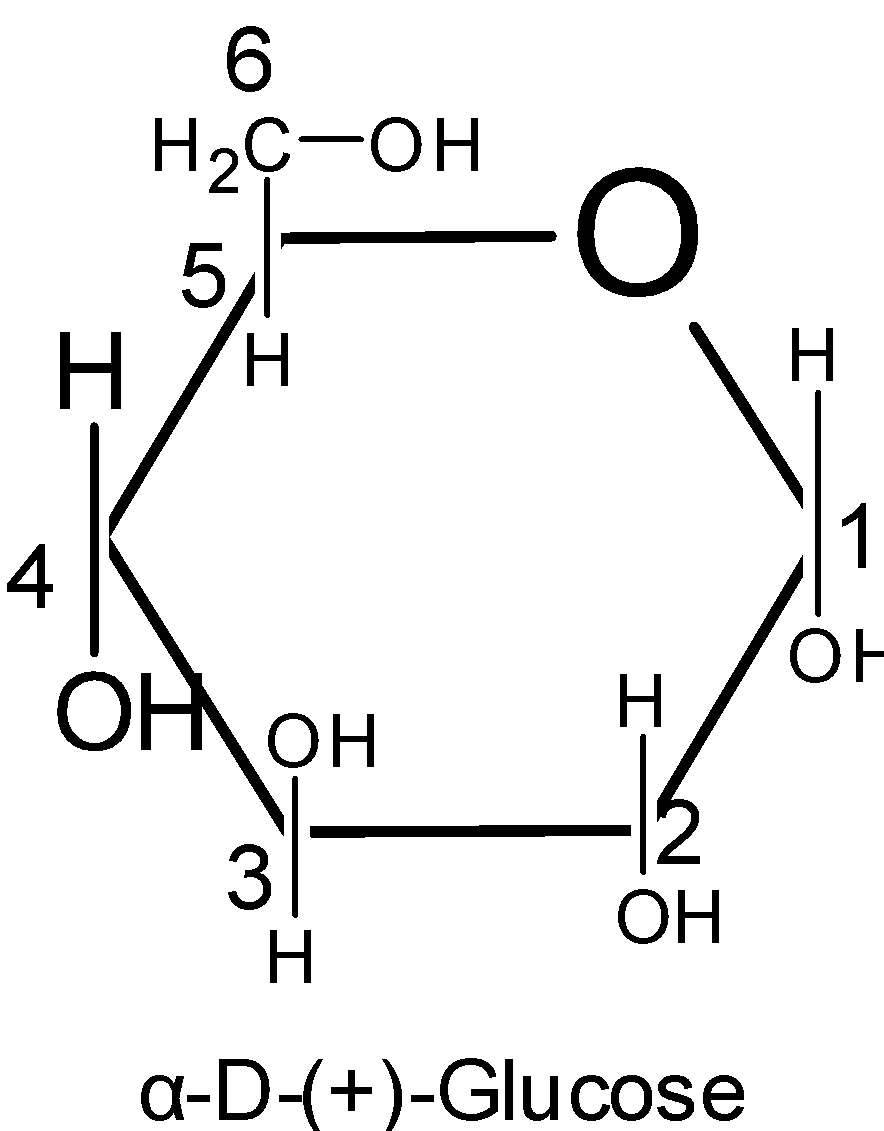
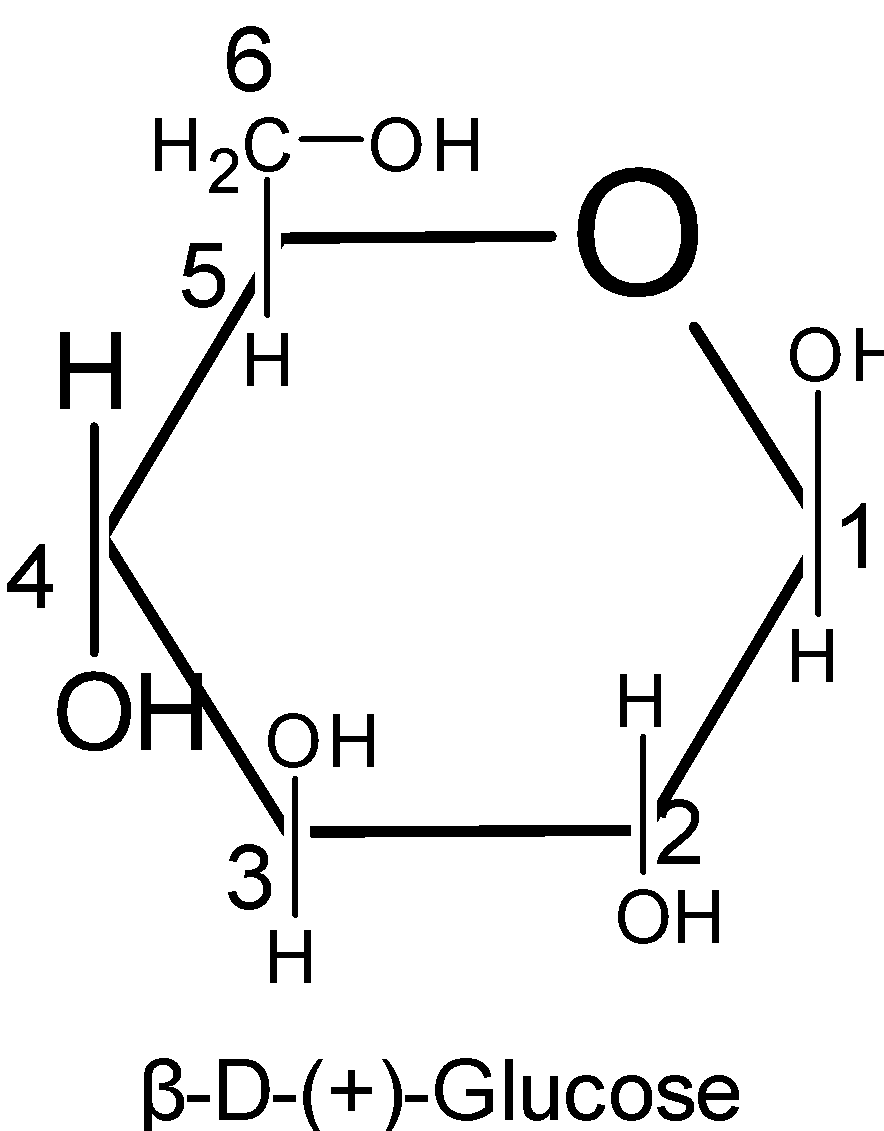
Thus here we know that the six membered rings of oxide are called the pyranose. Due to the formation of an oxide ring, the new asymmetric carbon atom is created at the carbonyl carbon, which is called an anomeric carbon atom.
Two different configurations are possible for the anomeric carbon atom and they are called the anomers.
So, the correct answer is “Option C”.
Note: Remember that groups which are on the right in a Fischer projection are written down in Haworth projection and groups which are on the left in a Fischer projection are up in a Haworth projection.
Complete step by step answer:
Pyranose is a term used for the saccharides that have the chemical structure. It is a chemical structure for the six-membered rings which consist of five carbon atoms and one oxygen atom in it. There can be other external carbons to the ring.
The structure of $\text{ }\!\!\alpha\!\!\text{ -D-glucose}$ and $\text{ }\!\!\beta\!\!\text{ -D-glucose}$ may be drawn in a simple six-membered ring which is called pyranose structures. These structures resemble the pyran which is a six-membered heterocyclic ring containing five carbon atoms and one oxygen atom.

These structures were suggested by Haworth and are known as the Haworth projection formulae or pyranose structures.
To write the pyranose structures for any monosaccharide$\text{( }\!\!\alpha\!\!\text{ -and }\!\!\beta\!\!\text{ -D-glucose)}$, first draw a hexagon with its oxygen atom at the upper right-hand corner. The terminal $\text{-C}{{\text{H}}_{\text{2}}}\text{OH}$ group as shown in the figure is always placed above the plane of the hexagon ring. Place all the groups which are present on the left-hand side in Fischer projection above the plane of the ring and all those groups on the right hand in Fischer projection below the plane of the ring.
Pyranose rings are formed by the reaction of the hydroxyl group on the carbon number five of sugar with the aldehyde which is at the carbon one. This formation goes through the hemiacetal.


Thus here we know that the six membered rings of oxide are called the pyranose. Due to the formation of an oxide ring, the new asymmetric carbon atom is created at the carbonyl carbon, which is called an anomeric carbon atom.
Two different configurations are possible for the anomeric carbon atom and they are called the anomers.
So, the correct answer is “Option C”.
Note: Remember that groups which are on the right in a Fischer projection are written down in Haworth projection and groups which are on the left in a Fischer projection are up in a Haworth projection.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




