
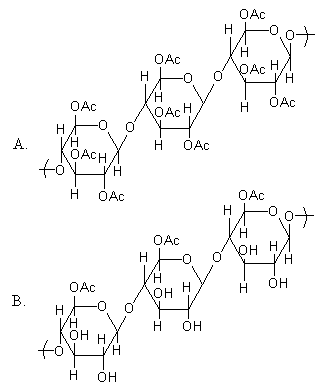
Cellulose upon acetylation with excess acetic anhydride/ ${{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}$ (catalytic) gives cellulose triacetate whose structure is:


Answer
563.1k+ views
Hint: To answer this question we should know the structure of cellulose, its structural unit and bonding between structural units. Acetylation is the conversion of an alcoholic group into an acetyl group. So, we will draw the structure of the cellulose and then we will replace the every –OH group into acetyl group to obtain the structure of triacetate. As we obtain the triacetate so, we will draw only three structural units of cellulose.
Complete solution:
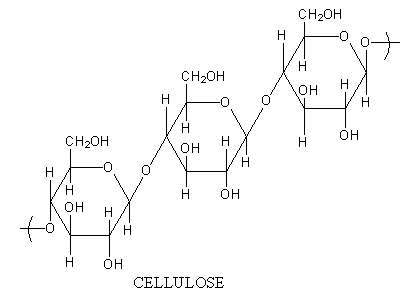
Cellulose is linear chains of $\beta $-D-glucose molecules linked together by$\beta - 1,4$glycosidic bonds. Cellulose has $\beta - 1,4$ linkage between glucose units. The cellulose is a linear molecule.
A general reaction of alcohol with acetic anhydride is shown as follows:
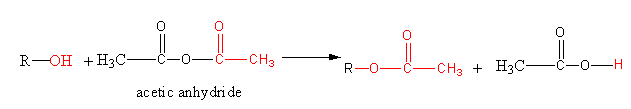
Acetic anhydride reacts with the alcoholic function group and converts the alcoholic function group into ester functional group. The –OH is known as an alcoholic functional group. The $ - {\text{OC(O}}) - $ is known as an ester functional group. During the conversion hydrogen of alcoholic group is replaced by ${\text{C}}{{\text{H}}_{\text{3}}}{\text{CO}} - $ group of acetic anhydride. The above reaction is known as acetylation because acetyl group ${\text{C}}{{\text{H}}_{\text{3}}}{\text{CO}} - $ is attaching with the reactant alcohol.
So, upon acetylation of cellulose with excess acetic anhydride/ ${{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}$ (catalytic) all the –OH group will convert into acetyl group.
As the cellulose contains a large number of glucose units but our product is triacetate. It means the alcohol group of three glucose units of cellulose is converted into acetyl group. As the acetic anhydride is in excess, all alcoholic groups of three glucose units of cellulose will convert into acetyl groups.
So, the structure of the product is,
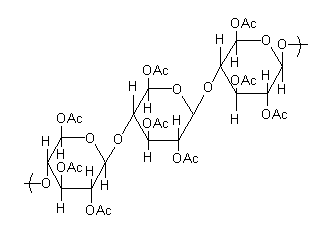
Each glucose unit of cellulose has three alcoholic groups, so a total of nine alcoholic groups convert into acetyl to give triacetate. The above structure is triacetate of cellulose.
Therefore, option (A) is correct.
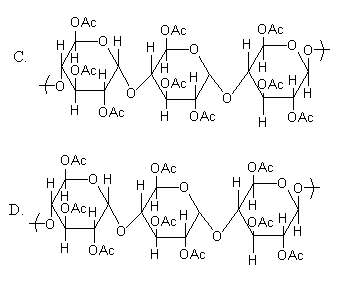
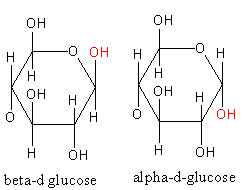
Note: The option (C) also has acetyl group in place of each –OH group but the given structure is not correct. Because the structure given in option (C) corresponds to the formation of cellulose by alpha-d-glucose whereas the cellulose has alpha-d-glucose. The difference between alpha and beta d-glucose is shown as follows:
Complete solution:
Cellulose is linear chains of $\beta $-D-glucose molecules linked together by$\beta - 1,4$glycosidic bonds. Cellulose has $\beta - 1,4$ linkage between glucose units. The cellulose is a linear molecule.

A general reaction of alcohol with acetic anhydride is shown as follows:

Acetic anhydride reacts with the alcoholic function group and converts the alcoholic function group into ester functional group. The –OH is known as an alcoholic functional group. The $ - {\text{OC(O}}) - $ is known as an ester functional group. During the conversion hydrogen of alcoholic group is replaced by ${\text{C}}{{\text{H}}_{\text{3}}}{\text{CO}} - $ group of acetic anhydride. The above reaction is known as acetylation because acetyl group ${\text{C}}{{\text{H}}_{\text{3}}}{\text{CO}} - $ is attaching with the reactant alcohol.
So, upon acetylation of cellulose with excess acetic anhydride/ ${{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}$ (catalytic) all the –OH group will convert into acetyl group.
As the cellulose contains a large number of glucose units but our product is triacetate. It means the alcohol group of three glucose units of cellulose is converted into acetyl group. As the acetic anhydride is in excess, all alcoholic groups of three glucose units of cellulose will convert into acetyl groups.
So, the structure of the product is,

Each glucose unit of cellulose has three alcoholic groups, so a total of nine alcoholic groups convert into acetyl to give triacetate. The above structure is triacetate of cellulose.
Therefore, option (A) is correct.
Note: The option (C) also has acetyl group in place of each –OH group but the given structure is not correct. Because the structure given in option (C) corresponds to the formation of cellulose by alpha-d-glucose whereas the cellulose has alpha-d-glucose. The difference between alpha and beta d-glucose is shown as follows:

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




